NewAmsterdam Pharma Reports Positive Results from BROOKLYN Phase 3 Trial on Obicetrapib in Heterozygous Familial Hypercholesterolemia
NewAmsterdam Pharma Company N.V., a biopharmaceutical firm in the advanced stages of clinical development, focusing on creating oral, non-statin treatments for individuals at risk of cardiovascular disease with high levels of low-density lipoprotein cholesterol, for whom current treatments are either inadequately effective or poorly tolerated, has reported promising topline results from its Phase 3 BROOKLYN clinical study.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The initial study within NewAmsterdam's key clinical development series, titled BROOKLYN, aimed to assess obicetrapib in adult individuals with heterozygous familial hypercholesterolemia, who have inadequately controlled LDL-C despite maximal lipid-lowering therapies.
"My professional focus has been the management of patients with familial hypercholesterolemia," stated John Kastelein, M.D., Ph.D., FESC, Chief Scientific Officer at NewAmsterdam. "Many of these patients have exhausted current treatment options, and we are pleased with the topline results from BROOKLYN, showing 51% of participants achieving LDL-C levels under 70 mg/dl. Our objective is to offer doctors and patients a novel, once-daily, low-dose oral option that could significantly alter the treatment paradigm."
"The findings revealed today further highlight obicetrapib's potential to considerably lower LDL-C in HeFH patients who are already on multiple lipid-lowering treatments. These results are very encouraging, indicating that obicetrapib, pending approval, could offer a new oral treatment for a challenging patient group. I am enthusiastic about continuing the collaboration with the NewAmsterdam team throughout the rest of the obicetrapib pivotal studies," noted Stephen Nicholls, M.B.B.S., Ph.D., Director of the Monash Victorian Heart Institute and Professor of Cardiology at Monash University.
"HeFH impacts 1 in 250 individuals worldwide, increasing the risk of major adverse cardiovascular events, such as stroke, myocardial infarction, or death, during the prime of life due to chronic high LDL-C levels. Despite existing treatment options, many people with HeFH fail to meet guideline-recommended LDL-C targets," said Katherine Wilemon, Founder and CEO of the Family Heart Foundation. "HeFH usually necessitates multiple therapies to achieve safer LDL cholesterol levels. We are greatly encouraged by these results and the possibility of a new effective oral option."
Obicetrapib is an innovative, oral, low-dose CETP inhibitor being developed by NewAmsterdam to address the limitations of existing LDL-lowering treatments. In the company's Phase 2 trials (ROSE2, TULIP, ROSE, and OCEAN) and the Phase 3 BROOKLYN trial, assessing obicetrapib alone or in combination therapy, statistically significant LDL-lowering effects were observed alongside a placebo-like side effect profile.
In April 2024, NewAmsterdam finalized enrollment for the PREVAIL study, randomizing over 9,500 patients. The Menarini Group, a leading international pharmaceutical and diagnostics company based in Italy, has been granted exclusive commercialization rights for obicetrapib in Europe, either as a monotherapy or in a fixed dose combination with ezetimibe, for cardiovascular conditions.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
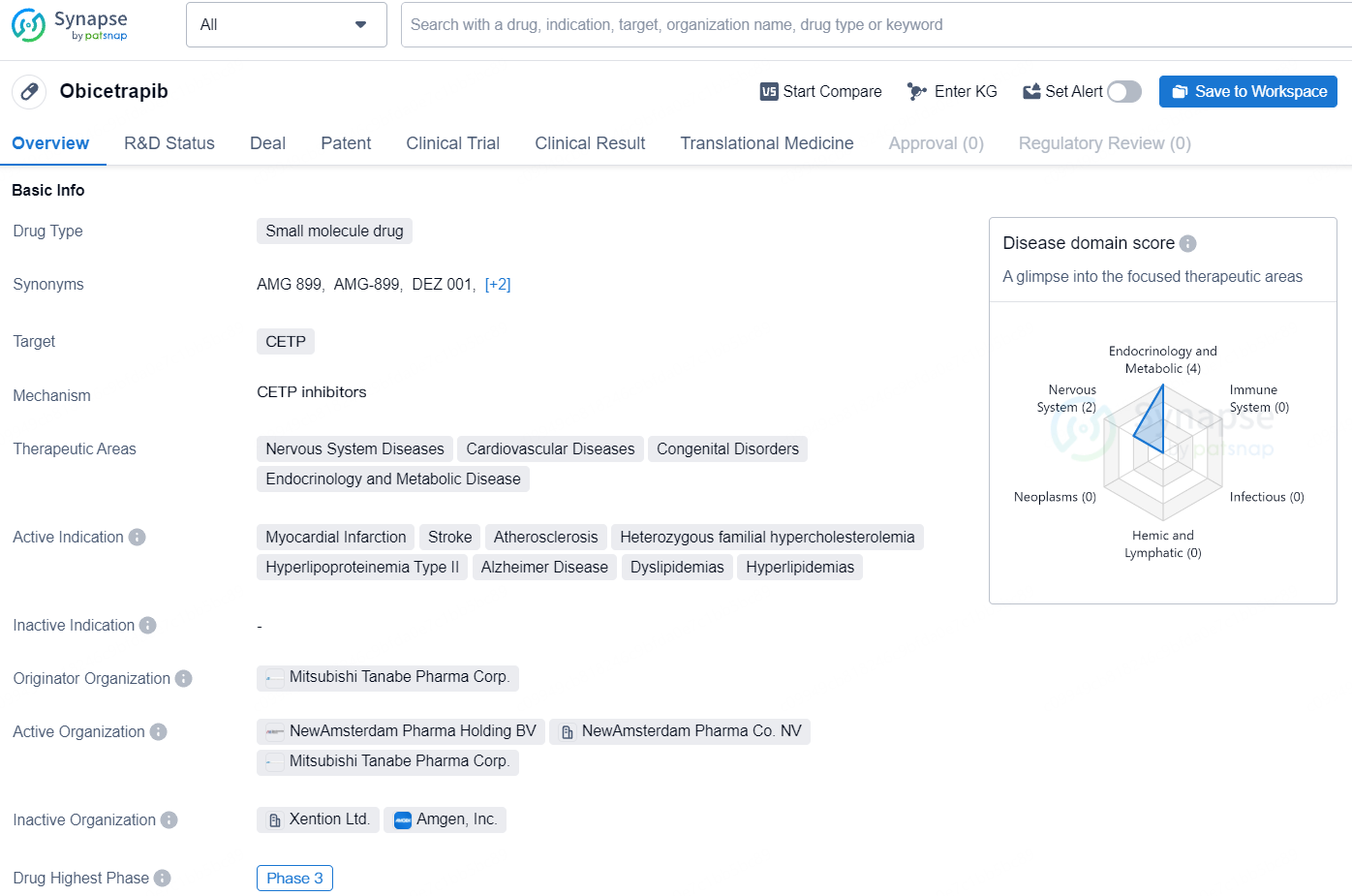
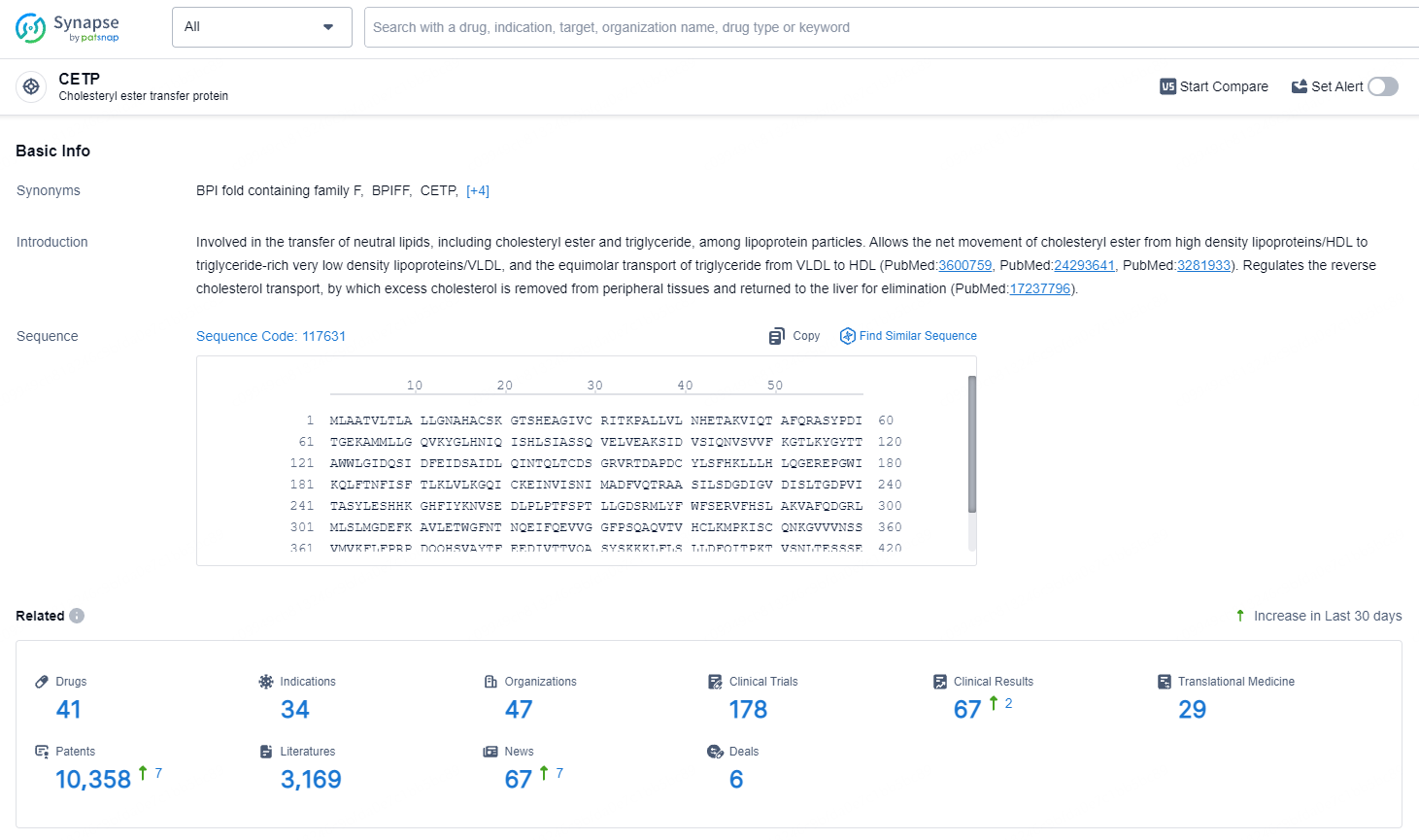
According to the data provided by the Synapse Database, As of August 1, 2024, there are 41 investigational drugs for the CETP target, including 34 indications, 47 R&D institutions involved, with related clinical trials reaching 178, and as many as 10358 patents.
Obicetrapib is a small molecule drug designed to target CETP for the treatment of various diseases related to the nervous system, cardiovascular system, congenital disorders, and metabolic diseases. The drug has shown promising potential as it has advanced to the highest phase of clinical development, Phase 3, both globally and in China. These significant milestones indicate that Obicetrapib is progressing towards potential regulatory approval and eventual commercialization.