Alpine Immune Sciences Initiates Administration of 240mg IgA Nephropathy Group in Povetacicept Clinical Trial (RUBY-3)
Alpine Immune Sciences, Inc., a prominent clinical-stage firm concentrating on the development of breakthrough therapies for autoimmune and inflammatory conditions, has revealed the successful launch of the second IgA nephropathy (IgAN) dosage group in RUBY-3, a phase 1b/2a.
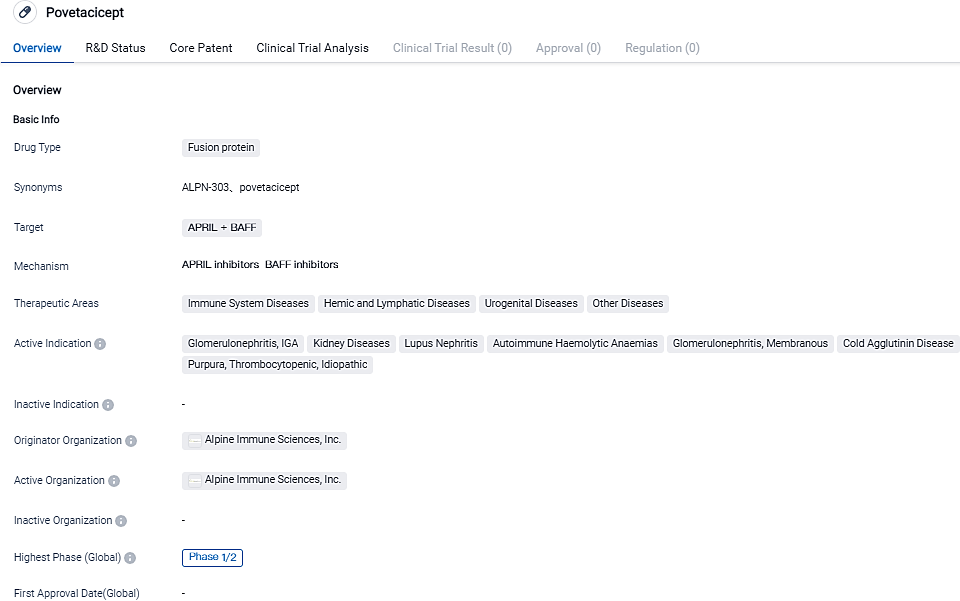
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
• The Safety Monitoring Committee has concluded that the repeated usage of povetacicept in the initial IgAN dose group (80 mg injected beneath the skin every four weeks) has thus far proven safe and easily tolerated, with no serious or severe side effects, no incidents of hypogammaglobulinemia (IgG < 3g/L), and no cases of hypersensitivity or reactions related to administration.
• The committee has approved an increase in dosage, and registration of the second IgAN dose group (240 mg injected subcutaneously every four weeks) has begun.
"The preliminary safety and pharmacodynamic performance during multiple dose administration of povetacicept, at its lowest dosage in this trial, has offered us cause for optimism," observed Stanford Peng, the President and the head of R&D at Alpine. "We anticipate sharing a more in-depth clinical update in suitable academic gatherings later this year."
About Povetacicept (ALPN-303)
Povetacicept (ALPN-303) is an antagonist that counteracts both BAFF + APRIL. These cytokines are instrumental to the development of several autoimmune conditions through their effects on the activation, differentiation, and/or survival of B cells, especially cells that make antibodies, along with T cells and cells that form the innate immune system. Utilizing a restructured TACI domain, povetacicept has shown greater effectiveness in experimental trials than natural TACI counterparts, as well as other inhibitors of BAFF and/or APRIL. Povetacicept is currently undergoing development for several autoimmune diseases like systemic lupus erythematosus, autoimmune glomerulonephritis, and autoimmune cytopenias.
About RUBY-3
RUBY-3 is a phased 1b/2a clinical study with escalating dosage, different groups, and an open label. It is analyzing the effects of povetacicept on IgA nephropathy, lupus nephritis, and primary membranous nephropathy, where the drug is administered subcutaneously for a maximum duration of 48 weeks. Key conclusions of this study include proteinuria, eGFR, renal response, and disease-specific autoantibodies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the information disclosed by the Synapse Database, as of September 1, 2023, there are 4 investigational drugs for the BAFF + APRIL target, including 22 applicable indications, 11 R&D institutions involved, with related clinical trials reaching 85, and as many as 2118 patents.Alpine Immune Sciences is committed to leading a new wave of immune therapeutics. With world-class research and development capabilities, a highly productive scientific platform, and a proven management team, Alpine is seeking to create first- or best-in-class multifunctional immunotherapies via unique protein engineering technologies to improve patients’ lives. Alpine has entered into strategic collaborations with leading global biopharmaceutical companies and has a diverse pipeline of clinical and preclinical candidates in development.