Alvotech Gives U.S. Regulatory Progress Report on AVT02, a Humira® (adalimumab) Interchangeable Biosimilar Candidate with High Concentration
Alvotech, an international biotechnology company that focuses on creating and producing biosimilar drugs for global patients, declared today that the U.S. FDA has agreed to Alvotech's revised BLA for AVT02. This medicine is a highly concentrated, substitutable biosimilar alternative to Humira®. Additionally, the FDA has appointed a Biosimilar User Fee Act target date for the authorization of the reproposed AVT02 BLA
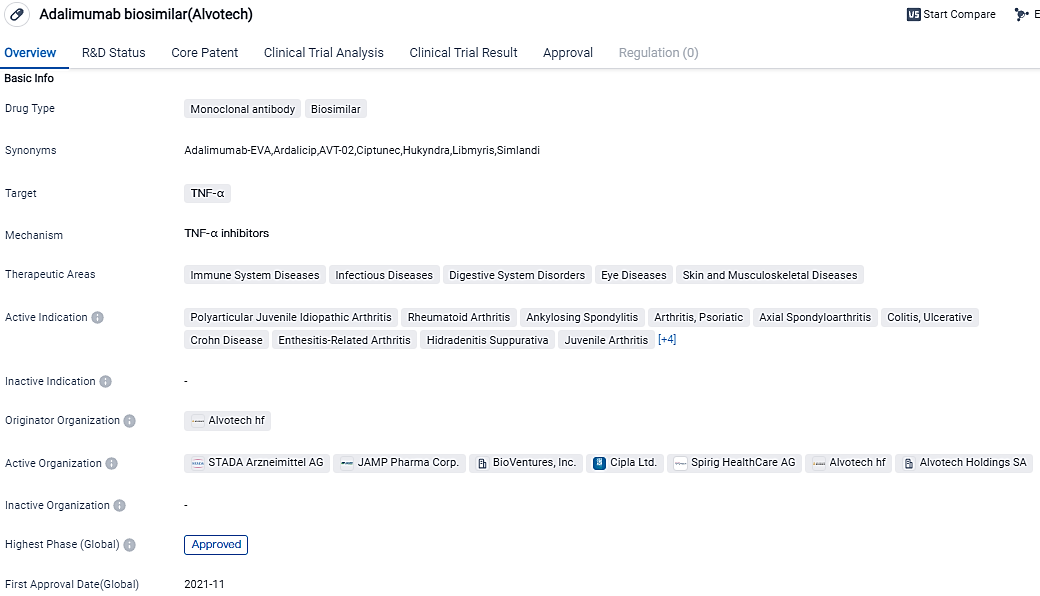
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The FDA has set the BsUFA target date for February 24, 2024. It has informed Alvotech that the company's resubmission is viewed as a comprehensive reply to the agency’s action letter of June 28, 2023. This appreciation is on account of the supplementary Chemistry, Manufacturing, and Controls details presented by the corporation in the BLA to rectify manufacturing infrastructure shortcomings previously pointed out by the FDA.
Robert Wessman, Chairman and CEO of Alvotech, stated their dedicated purpose of introducing AVT02 to the U.S. medical market, where there remains a substantial and unsatisfied need for an interchangeable biosimilar to Humira in high concentration. He added that they look forward to additional instructions from the FDA regarding the timeframe for a re-inspection, which they anticipate happening prior to the BsUFA deadline.
An interchangeable biosimilar has the potential to be replaced at the pharmacy without needing the authorisation of the healthcare expert who originally prescribed the biologic drug, despite possible differences in local pharmacy regulations and practices. As of now, these biosimilars of adalimumab in high concentration are not accessible in the U.S.
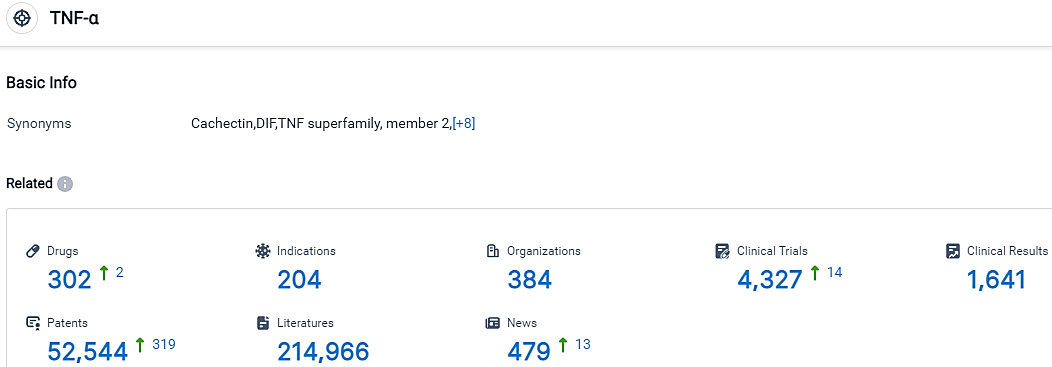
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 302 investigational drugs for the TNF-α target, including 204 indications,384 R&D institutions involved, with related clinical trials reaching 4327,and as many as 52544 patents.
AVT02, a monoclonal antibody, has been greenlit worldwide as a biosimilar to Humira® (adalimumab) in numerous regions. This includes 27 countries within the European Union, Norway, Lichtenstein, Iceland, the United Kingdom, Switzerland, Canada, Australia, Egypt, and Saudi Arabia. At the moment, it's being sold in several nations across Europe, as well as Canada. The approval documents for its use are presently being evaluated in several countries.