Exploring Prednisolone Sodium Phosphate's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Prednisolone Sodium Phosphate's R&D Progress
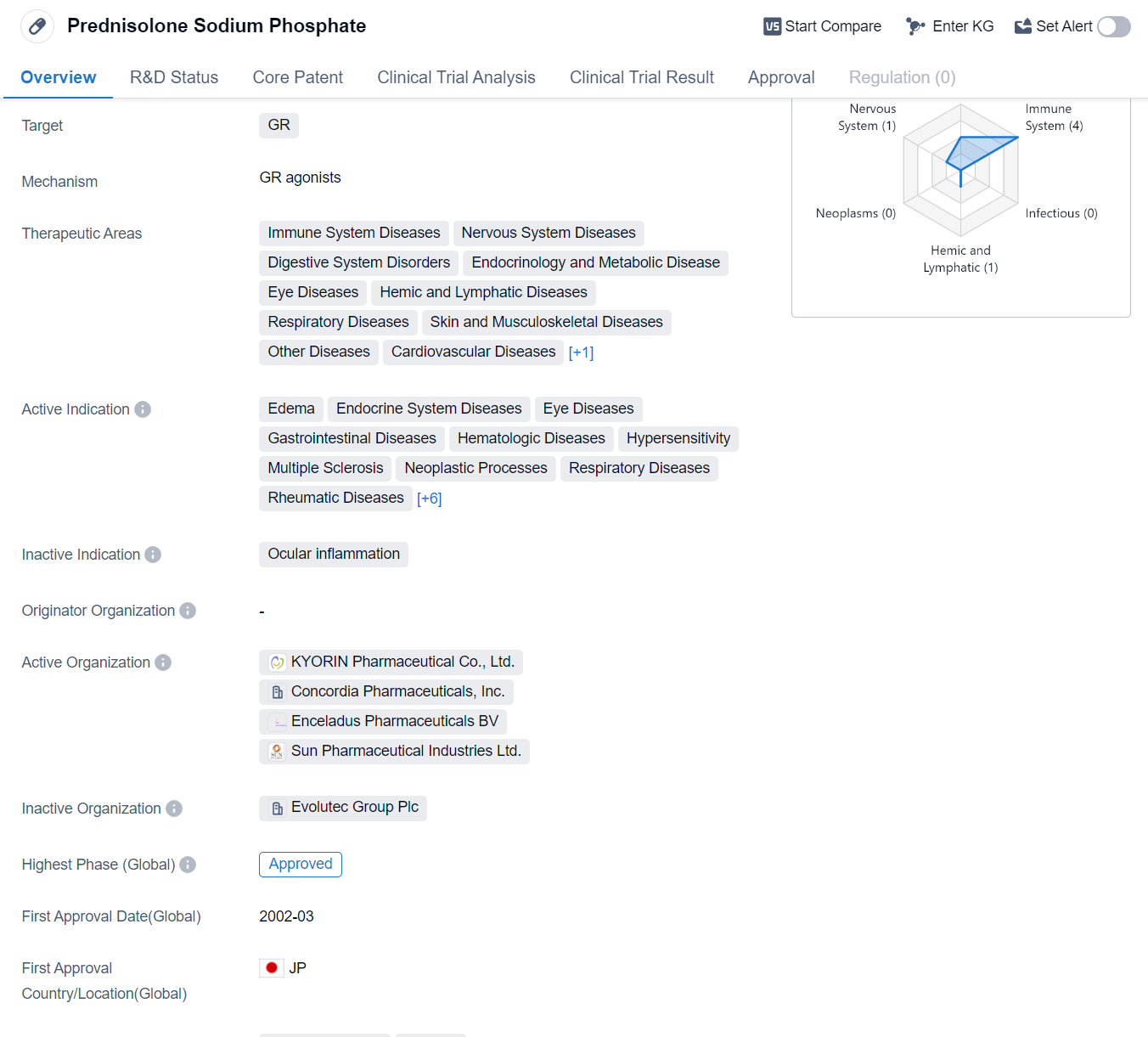
Prednisolone Sodium Phosphate is a small molecule drug that primarily targets the glucocorticoid receptor (GR). It has been approved for use in various therapeutic areas, including immune system diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic diseases, eye diseases, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases,cardiovascular diseases, and congenital disorders.
The active indications for Prednisolone Sodium Phosphate are diverse and include edema, endocrine system diseases, eye diseases, gastrointestinal diseases, hematologic diseases, hypersensitivity, multiple sclerosis, neoplastic processes, respiratory diseases, rheumatic diseases, skin diseases, colitis, ulcerative, enteritis, rheumatoid arthritis, Graves ophthalmopathy, and arteriovenous fistula.
The highest R&D phase of this drug is approved.. It received its first approval in Japan in March 2002.
As a small molecule drug targeting the GR, Prednisolone Sodium Phosphate is likely to exert its effects by modulating the activity of the glucocorticoid receptor. This receptor plays a crucial role in regulating various physiological processes, including immune responses, metabolism, and stress responses. By targeting the GR, Prednisolone Sodium Phosphate may help to alleviate symptoms and manage diseases associated with dysregulation in these pathways.
The broad range of therapeutic areas and active indications for Prednisolone Sodium Phosphate highlights its versatility and potential for treating a wide range of diseases. Its approval in Japan suggests that it has demonstrated significant clinical benefits and met the necessary regulatory requirements in this country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Prednisolone Sodium Phosphate: GR agonist
From a biomedical perspective, GR agonists refer to substances that activate the glucocorticoid receptor (GR). The GR is a nuclear receptor that plays a crucial role in regulating various physiological processes, including immune response, metabolism, and stress response. When a GR agonist binds to the GR, it initiates a cascade of signaling events that ultimately lead to changes in gene expression. This can result in anti-inflammatory effects, immunosuppression, and modulation of glucose metabolism. GR agonists are commonly used in the treatment of conditions such as asthma, autoimmune disorders, and certain types of cancer. They can be administered orally, topically, or through inhalation, depending on the specific indication and formulation. It is important to note that the use of GR agonists should be carefully monitored, as prolonged or excessive activation of the GR can lead to adverse effects such as immunosuppression, osteoporosis, and metabolic disturbances.
Drug Target R&D Trends for Prednisolone Sodium Phosphate
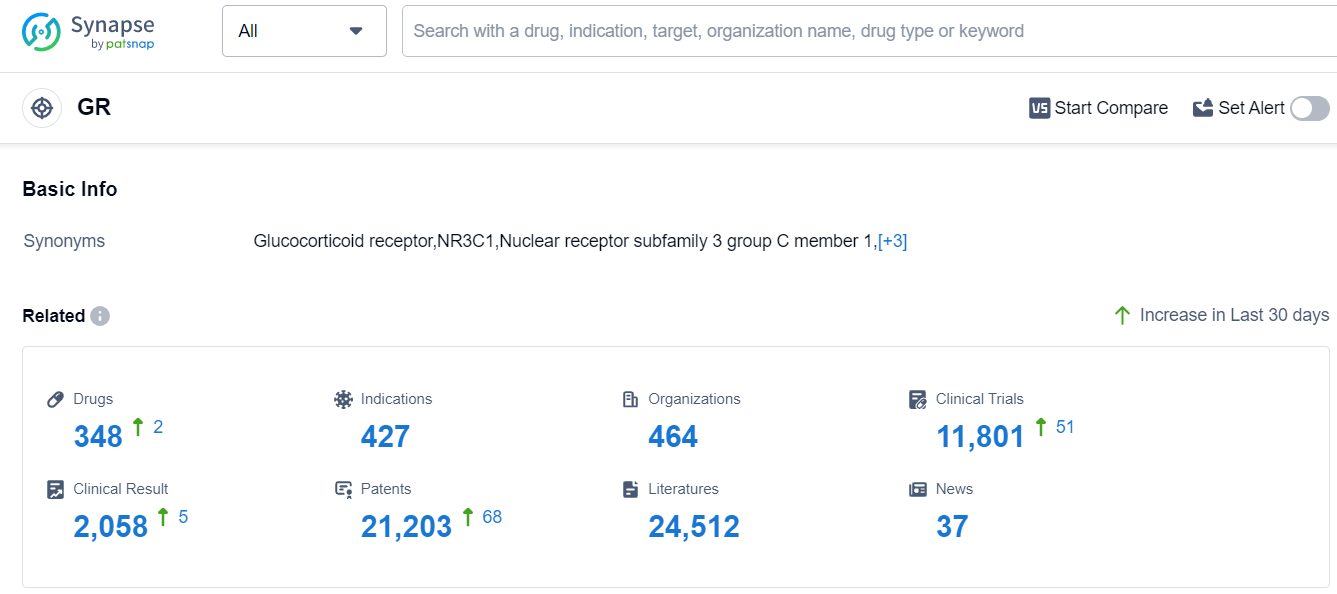
The analysis of the target GR reveals a competitive landscape with several companies showing growth and development in this area. Novartis AG, Bausch Health Cos., Inc., and Shionogi & Co., Ltd. are among the top companies with a diverse pipeline and approved drugs. Eczema, asthma, psoriasis, and skin diseases are the indications with the highest number of approved drugs, indicating a focus on these areas. Small molecule drugs dominate the drug types, suggesting intense competition and innovation. The United States and China are the leading countries in terms of development, with significant progress in both regions. China, in particular, has emerged as a strong player in the target GR. Overall, the target GR presents opportunities for further research, development, and competition in the pharmaceutical industry.
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 348 GR drugs worldwide, from 464 organizations, covering 427 indications, and conducting 11801 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Prednisolone Sodium Phosphate is an approved small molecule drug that targets the GR and has a diverse range of therapeutic applications. Its first approval in Japan in 2002 signifies its successful development and validation as a treatment option for various diseases. Further research and clinical studies may continue to explore its potential in managing and improving outcomes for patients with the indicated conditions.