Pharmaceutical Insights: Phendimetrazine Tartrate's R&D Progress
Phendimetrazine Tartrate's R&D Progress
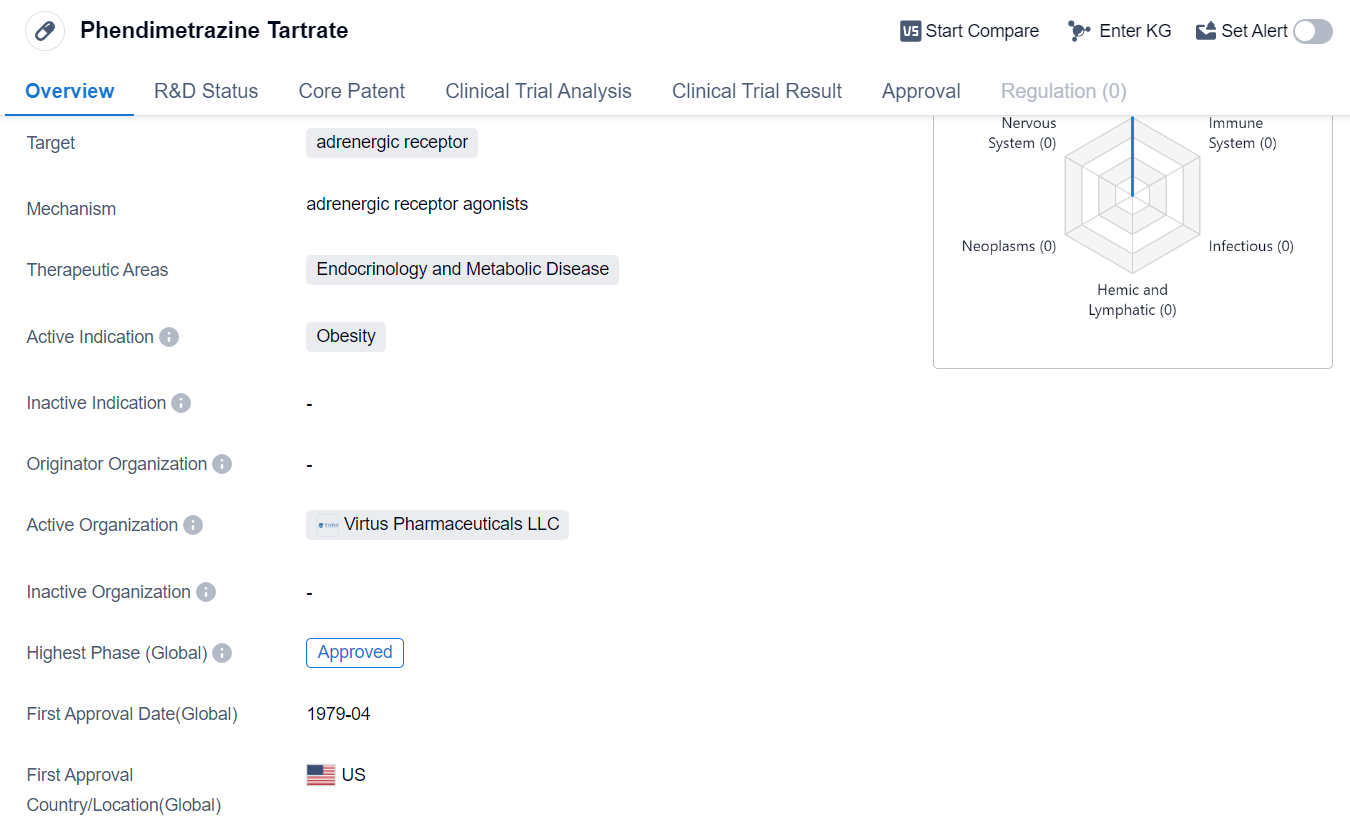
Phendimetrazine Tartrate is a small molecule drug that targets the adrenergic receptor. It falls under the therapeutic areas of Endocrinology and Metabolic Disease, with its active indication being obesity. The drug has reached the highest phase of development, which is approval.
Phendimetrazine Tartrate received its first approval globally in April 1979. The United States was the first country to approve this drug.
As a small molecule drug, Phendimetrazine Tartrate is designed to interact with the adrenergic receptor. This receptor is involved in the body's response to stress and plays a role in regulating various physiological processes, including metabolism. By targeting this receptor, Phendimetrazine Tartrate aims to modulate the body's response to stress and potentially impact metabolic pathways related to obesity.
The therapeutic areas of Endocrinology and Metabolic Disease encompass a range of conditions related to hormonal imbalances and metabolic dysfunctions. Obesity, the active indication for Phendimetrazine Tartrate, is a complex condition characterized by excessive body fat accumulation. It is associated with various health risks, including cardiovascular diseases, diabetes, and certain types of cancer.
Phendimetrazine Tartrate's approval status indicates that it has successfully completed the necessary clinical trials and regulatory processes to be deemed safe and effective for use in treating obesity. The drug's approval in the United States in 1979 suggests that it has a long history of use in this country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Phendimetrazine Tartrate: adrenergic receptor agonist
Adrenergic receptor agonists are a class of drugs that bind to and activate adrenergic receptors in the body. Adrenergic receptors are a type of receptor found on the surface of cells in various tissues and organs. They are part of the sympathetic nervous system, which is responsible for the body's "fight or flight" response.
When adrenergic receptor agonists bind to these receptors, they mimic the effects of the neurotransmitter norepinephrine, which is released by the sympathetic nervous system. This leads to a range of physiological responses, including increased heart rate, elevated blood pressure, dilation of the airways, and increased blood flow to certain organs.
In biomedicine, adrenergic receptor agonists are commonly used to treat conditions such as asthma, hypertension (high blood pressure), and certain cardiac disorders. By activating adrenergic receptors, these drugs can help relax and open up the airways, reduce blood pressure, and improve cardiac function.
It's important to note that adrenergic receptor agonists can have different selectivity for the various subtypes of adrenergic receptors, such as alpha and beta receptors. This selectivity determines the specific effects and potential side effects of each drug. Overall, adrenergic receptor agonists play a crucial role in regulating various physiological processes and are valuable therapeutic agents in biomedicine.
Drug Target R&D Trends for Phendimetrazine Tartrate
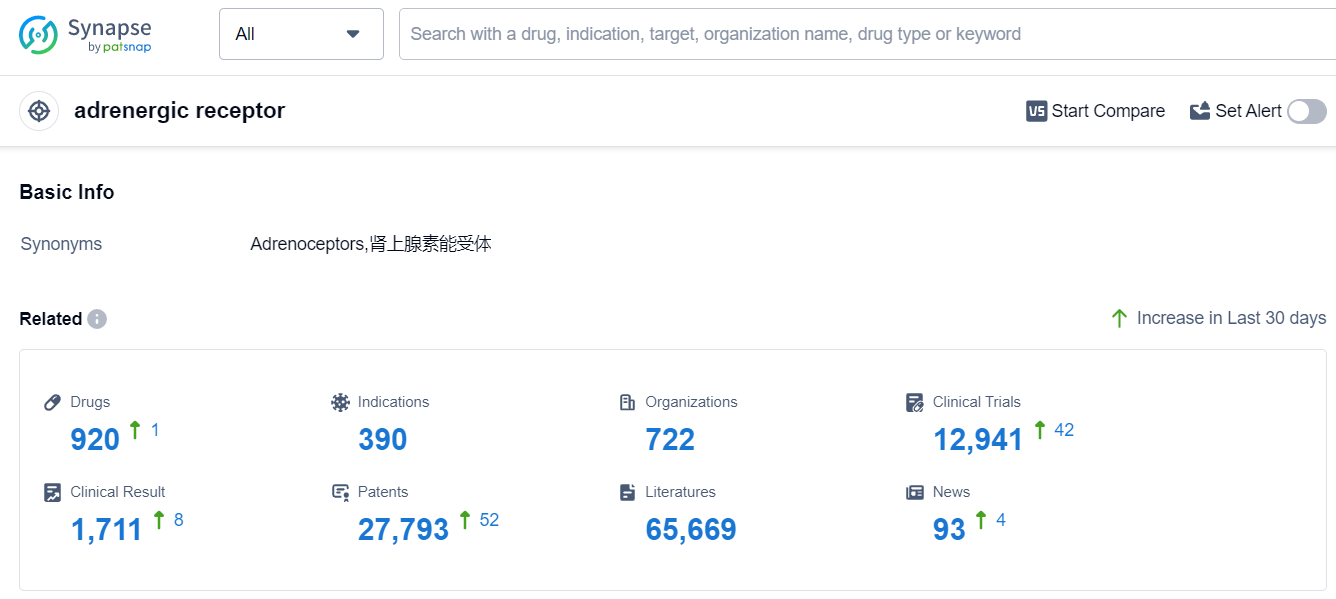
The analysis of the target adrenergic receptor reveals a competitive landscape with multiple companies actively involved in research and development. Novartis AG, GSK Plc, AstraZeneca PLC, Pfizer Inc., and Sanofi are the leading companies with a significant number of drugs in different development phases. Drugs targeting the adrenergic receptor have been approved for various indications, including hypertension, asthma, common cold, and pulmonary diseases. Small molecule drugs dominate the drug types, indicating their effectiveness and wide applicability. China, the United States, and Japan are the leading countries in terms of drug development, with China showing significant progress. Overall, the target adrenergic receptor presents a promising area for future development and therapeutic interventions in various diseases.
According to Patsnap Synapse, as of 17 Sep 2023, there are a total of 920 adrenergic receptor drugs worldwide, from 722 organizations, covering 390 indications, and conducting 12941 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Phendimetrazine Tartrate is a small molecule drug that targets the adrenergic receptor and is indicated for the treatment of obesity. Its approval status and long history of use in the United States highlight its potential efficacy and safety in addressing this prevalent health issue.