Decitabine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs, Mechanisms of Action, and Drug Target
Decitabine's R&D Progress
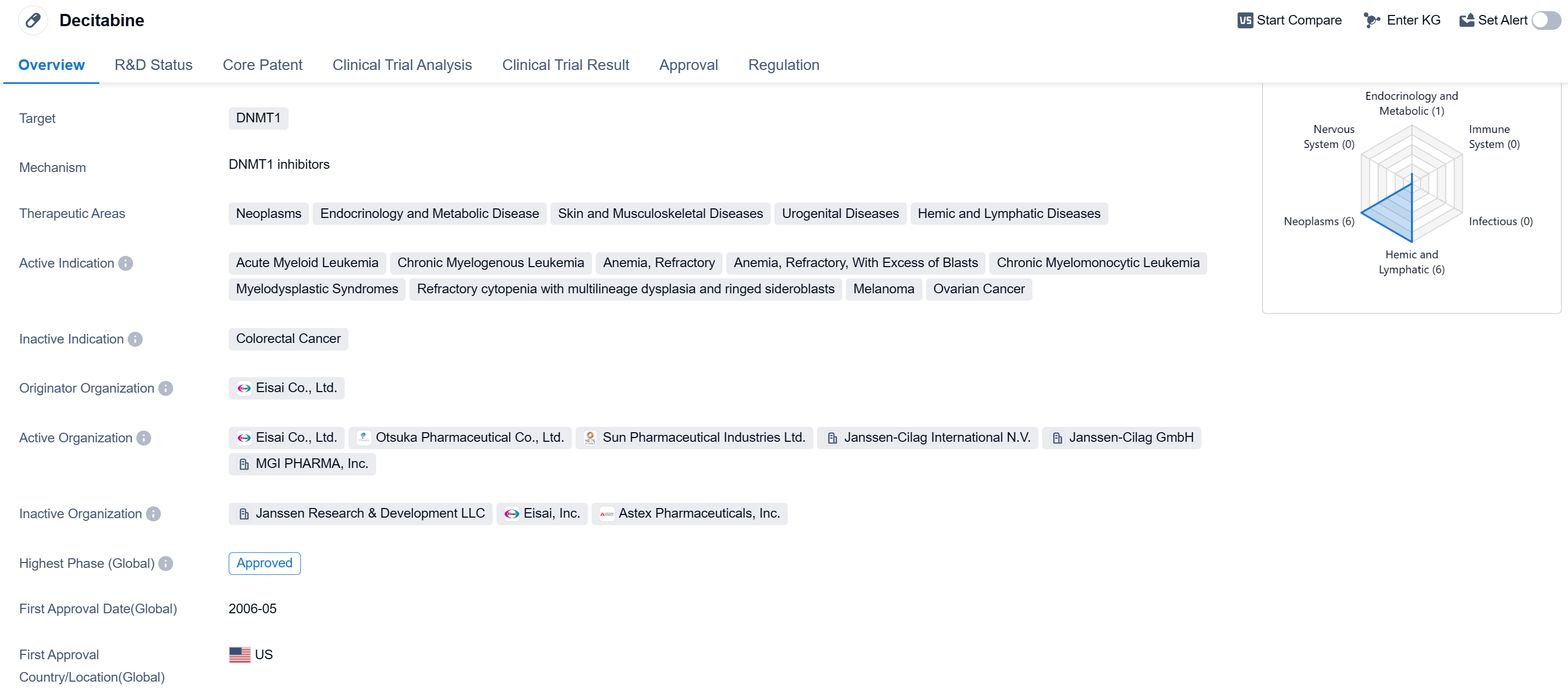
Decitabine is a small molecule drug that targets DNMT1, an enzyme involved in DNA methylation. It is used in the treatment of various neoplasms, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, and hemic and lymphatic diseases. The drug has been approved for multiple indications, including acute myeloid leukemia, chronic myelogenous leukemia, anemia refractory with excess of blasts, chronic myelomonocytic leukemia, myelodysplastic syndromes, refractory cytopenia with multilineage dysplasia and ringed sideroblasts, melanoma, and ovarian cancer.
Decitabine was developed by Eisai Co., Ltd., a pharmaceutical company. It received its first approval in the United States in May 2006 and has since been approved in other countries as well. The drug has reached the highest phase of development, which is approved globally.
One notable aspect of Decitabine is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives to the pharmaceutical company, such as market exclusivity and financial benefits, to encourage the development of drugs for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Decitabine: DNMT1 inhibitors
DNMT1 inhibitors are a type of drugs that target and inhibit the activity of DNA methyltransferase 1 (DNMT1), which is an enzyme involved in DNA methylation. DNA methylation is a process by which a methyl group is added to the DNA molecule, leading to changes in gene expression without altering the DNA sequence itself. DNMT1 is responsible for maintaining DNA methylation patterns during DNA replication, ensuring the inheritance of epigenetic information.
In the context of biomedicine, DNMT1 inhibitors have gained significant attention due to their potential therapeutic applications in various diseases, particularly cancer. Abnormal DNA methylation patterns, including hypermethylation of tumor suppressor genes and hypomethylation of oncogenes, are commonly observed in cancer cells. These aberrant DNA methylation patterns can contribute to the development and progression of cancer by altering gene expression.
By inhibiting DNMT1, DNMT1 inhibitors can disrupt the abnormal DNA methylation patterns in cancer cells, leading to the reactivation of tumor suppressor genes and the silencing of oncogenes. This can potentially restore normal gene expression and halt the growth and spread of cancer cells. DNMT1 inhibitors are being investigated as a promising therapeutic approach in cancer treatment, either as standalone agents or in combination with other anticancer drugs.
It is important to note that DNMT1 inhibitors may have off-target effects and can also affect other DNA methyltransferases, such as DNMT3A and DNMT3B. These enzymes are involved in de novo DNA methylation and play a role in normal development and cellular processes. Therefore, the development of selective DNMT1 inhibitors with minimal off-target effects is an active area of research in order to maximize the therapeutic benefits while minimizing potential side effects.
Drug Target R&D Trends for Decitabine
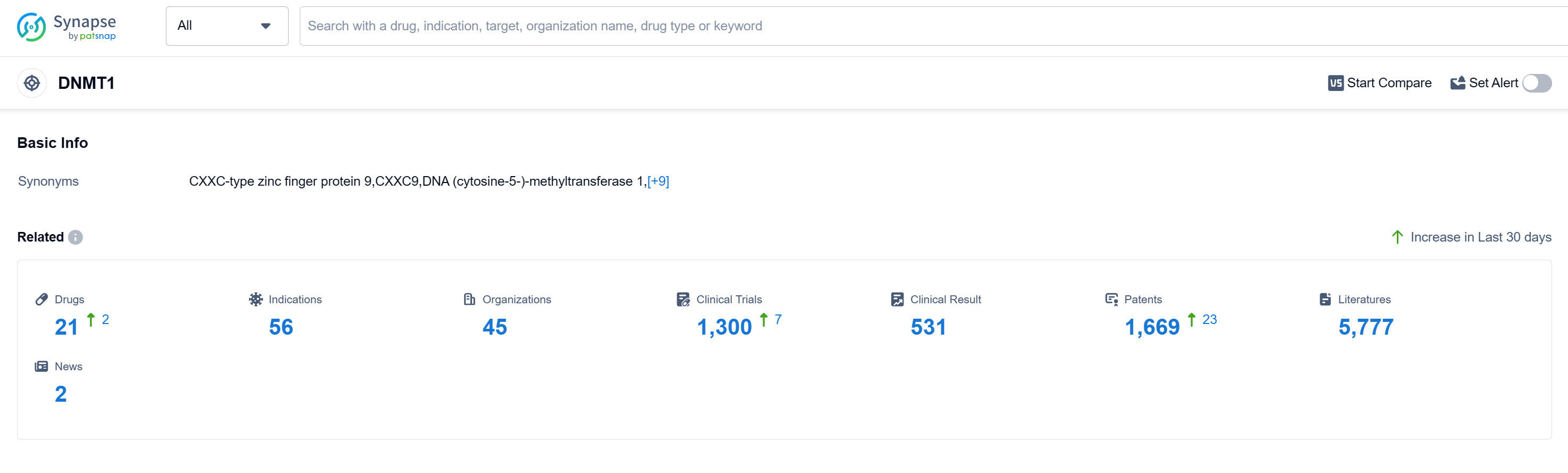
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 21 DNMT1 drugs worldwide, from 45 organizations, covering 56 indications, and conducting 1300 clinical trials.
The analysis of the current competitive landscape of target DNMT1 reveals that Otsuka Holdings Co., Ltd., Eisai Co., Ltd., Bristol Myers Squibb Co., Johnson & Johnson, and other companies are leading in terms of R&D progress. The highest stage of development is the "Approved" phase, with drugs approved for indications such as Myelodysplastic Syndromes, Chronic Myelomonocytic Leukemia, Acute Myeloid Leukemia, and others. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, China, European Union, and Japan are the countries/locations with the fastest development, with China showing significant progress. Overall, the target DNMT1 has a competitive landscape with a focus on small molecule drugs and a global presence in terms of R&D efforts.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Decitabine is a small molecule drug that targets DNMT1 and is used in the treatment of various diseases, including different types of leukemia, anemia, myelodysplastic syndromes, melanoma, and ovarian cancer. It was developed by Eisai Co., Ltd. and received its first approval in the United States in 2006. The drug has reached the highest phase of development globally. Its regulatory status as an orphan drug highlights its importance in treating rare diseases.