Unleashing the Power of Crisaborole: A Comprehensive Review on R&D Breakthroughs, Action Mechanisms, and Drug Target
Crisaborole's R&D Progress
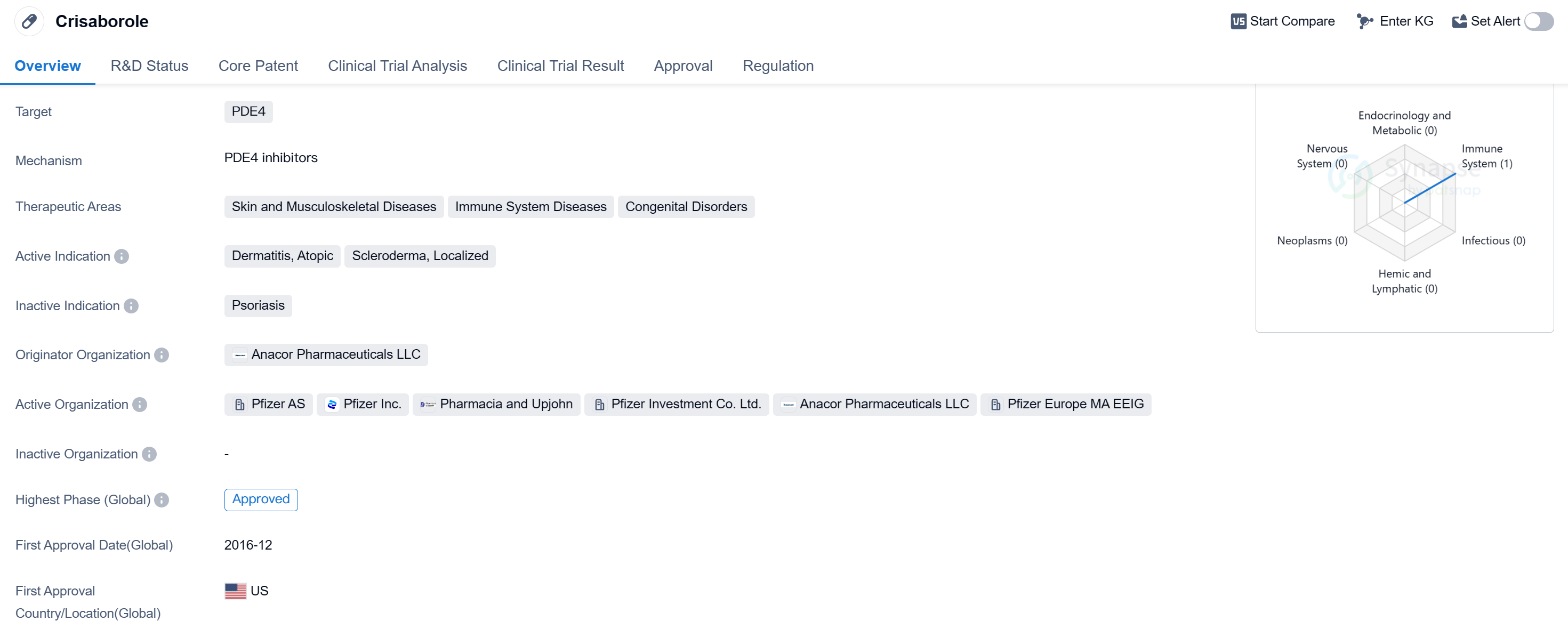
Crisaborole is a small molecule drug that targets PDE4 and is used in the treatment of various skin and musculoskeletal diseases, immune system diseases, and congenital disorders. The drug has been approved for use in the United States and China.
Crisaborole is primarily indicated for the treatment of dermatitis, atopic, scleroderma, and localized conditions. Dermatitis refers to inflammation of the skin, while atopic dermatitis is a chronic inflammatory skin disease characterized by itchy and inflamed skin. Scleroderma is a rare autoimmune disease that affects the skin and connective tissues, causing hardening and tightening. Localized conditions refer to specific areas of the body affected by skin disorders.
The drug was developed by Anacor Pharmaceuticals LLC, an originator organization specializing in the pharmaceutical industry. Crisaborole has successfully completed clinical trials and has received approval for use in both the United States and China. Its first approval was granted in December 2016 in the United States.
In terms of regulatory status, Crisaborole falls under the category of overseas new drugs urgently needed in clinical settings, which indicates its potential importance and demand in the medical field. Additionally, the drug has undergone priority review, suggesting that it has demonstrated significant therapeutic benefits and may address unmet medical needs.
Crisaborole's approval in both the United States and China highlights its potential as a treatment option for various skin and musculoskeletal diseases, immune system diseases, and congenital disorders. Its small molecule nature and targeting of PDE4 make it a promising candidate for further research and development in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Crisaborole: PDE4 inhibitors
PDE4 inhibitors are a type of drug that target and inhibit the enzyme phosphodiesterase 4 (PDE4). Phosphodiesterase enzymes play a crucial role in regulating the levels of cyclic adenosine monophosphate (cAMP) in cells. By inhibiting PDE4, these inhibitors prevent the breakdown of cAMP, leading to increased levels of cAMP in cells.
From a biomedical perspective, PDE4 inhibitors have shown therapeutic potential in various conditions, particularly in the field of respiratory medicine. They have been studied for their anti-inflammatory effects and their ability to relax smooth muscles in the airways. This makes them useful in the treatment of respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD).
By inhibiting PDE4, these drugs reduce the production of pro-inflammatory mediators and cytokines, leading to a decrease in airway inflammation. Additionally, they promote bronchodilation by relaxing the smooth muscles in the airways, which helps to alleviate symptoms of respiratory conditions.
PDE4 inhibitors are typically administered orally and are available in different forms, including tablets and capsules. Some examples of PDE4 inhibitors include roflumilast and cilomilast, which have been approved for the treatment of COPD in certain countries.
It's important to note that PDE4 inhibitors may have side effects, including gastrointestinal disturbances (such as nausea and diarrhea), headache, and potential interactions with other medications. Therefore, it is crucial to consult a healthcare professional before starting or changing any medication regimen involving PDE4 inhibitors.
Drug Target R&D Trends for Crisaborole
Additionally, PDE4 inhibitors have shown potential in treating respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD) by relaxing airway smooth muscles.
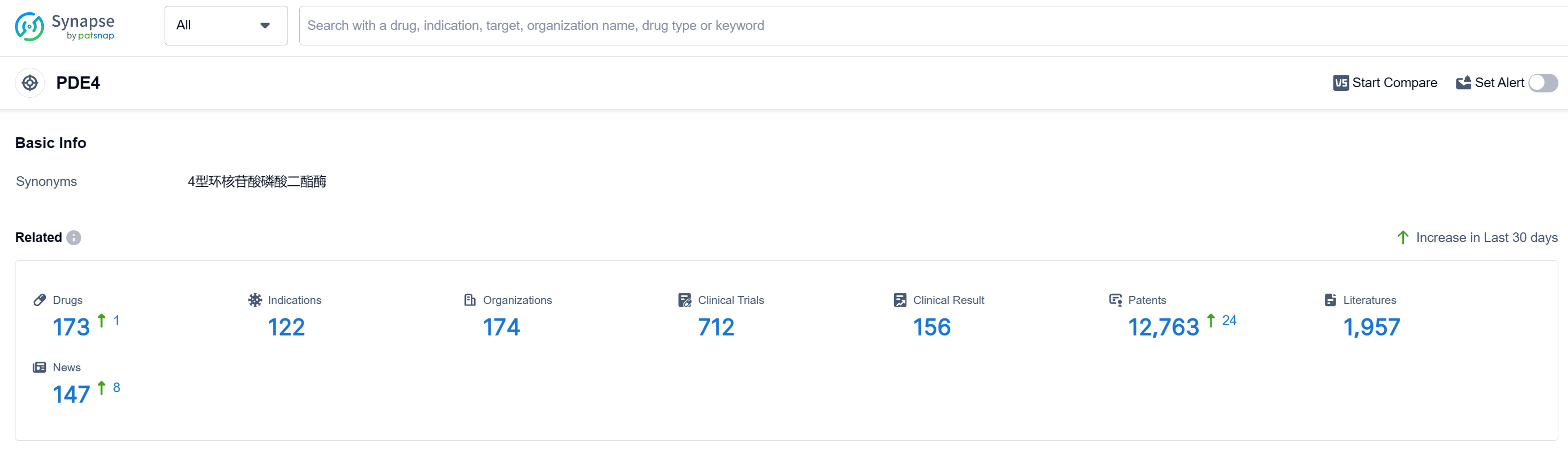
The analysis of the current competitive landscape of target PDE4 reveals that Pfizer Inc. is the leading company with the highest stage of development. The R&D progress of companies like Bristol Myers Squibb Co., Takeda Pharmaceutical Co., Ltd., and Arcutis Biotherapeutics, Inc. indicates a strong focus on developing drugs targeting PDE4. Drugs under the current target have been approved for various indications, showcasing the potential of PDE4 inhibitors in treating a wide range of diseases. Small molecule drugs and Diagnostic radiopharmaceuticals are progressing rapidly, indicating intense competition and potential innovation. China, the United States, and the European Union are the fastest-developing countries/locations, with China showing significant progress in PDE4 research and development. The future development of target PDE4 holds promise for advancements in the pharmaceutical industry and the treatment of various diseases.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Crisaborole is a small molecule drug developed by Anacor Pharmaceuticals LLC that targets PDE4. It has been approved for use in the treatment of dermatitis, atopic, scleroderma, and localized conditions. The drug has received first approvals in the United States and China, with its first approval granted in December 2016. Crisaborole's regulatory status as an overseas new drug urgently needed in clinical settings, along with its priority review, highlights its potential therapeutic benefits and importance in the pharmaceutical industry.