Amgen Reports Positive Phase 3 Outcomes for Uplizna® in Generalized Myasthenia Gravis at AANEM 2024
Amgen (NASDAQ:AMGN) reported promising top-line outcomes from the Phase 3 MINT study, which assessed the effectiveness and safety of UPLIZNA® (inebilizumab-cdon) in treating adults with generalized myasthenia gravis (gMG), a rare autoimmune condition. Details from this randomized, double-blind, placebo-controlled, parallel-group study will be shared at the Myasthenia Gravis Foundation of America (MGFA) Scientific Session, part of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting occurring from October 15-18 in Savannah, Georgia.
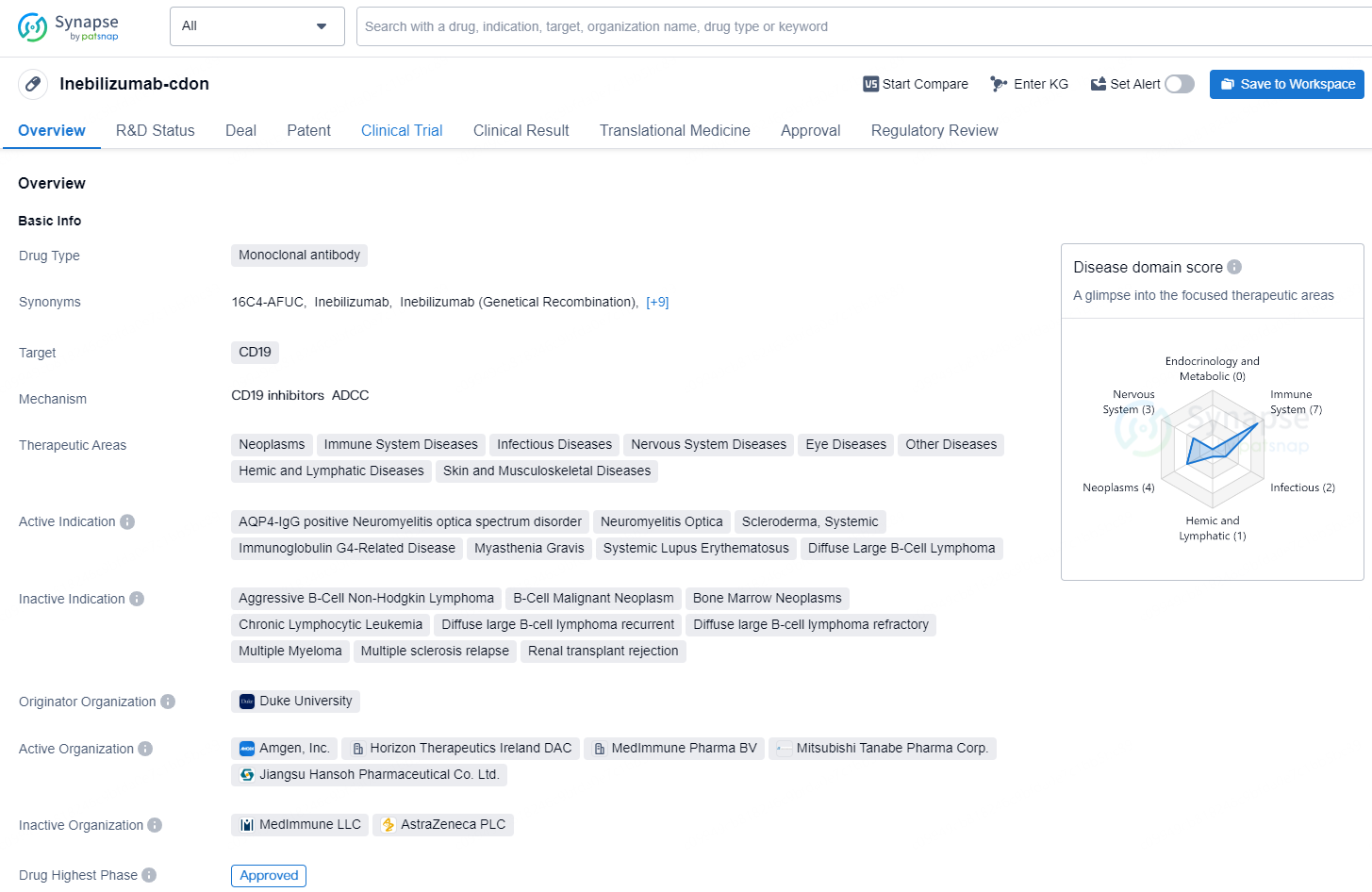
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial achieved its primary objective, showing a statistically significant improvement from baseline in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score for participants receiving UPLIZNA (-4.2) in comparison to those on placebo (-2.2) (difference: –1.9, p<0.0001) at the 26-week mark for the overall study group. Patients in the study included those positive for acetylcholine receptor autoantibodies (AChR+) as well as those positive for muscle-specific kinase autoantibodies (MuSK+), with treatment involving administration of UPLIZNA or placebo on Days 1 and 15. UPLIZNA continued to show progress up to Week 26. Importantly, participants who began the trial on corticosteroids had their dosage reduced starting Week 4, reaching a maintenance dose of prednisone 5 mg daily by Week 24. No new safety concerns emerged.
"Individuals with generalized myasthenia gravis need an effective therapeutic option that offers enduring symptom control. Once approved, UPLIZNA is anticipated to provide a novel option earlier in patients’ treatment protocols," stated Jay Bradner, M.D., executive vice president of Research and Development and chief scientific officer at Amgen. "UPLIZNA is designed to target CD19+ pre-B cells, mature B-cells, and certain plasmablasts, which are believed to contribute to the disease. The significant clinical outcomes from the MINT trial add to the already robust support for UPLIZNA in severe autoimmune conditions and affirm Amgen’s leading role in developing B-cell targeted treatments."
The main secondary endpoints were evaluated in a designated sequence. UPLIZNA exhibited a statistically significant and clinically relevant change from baseline compared to placebo for the primary four secondary endpoints.
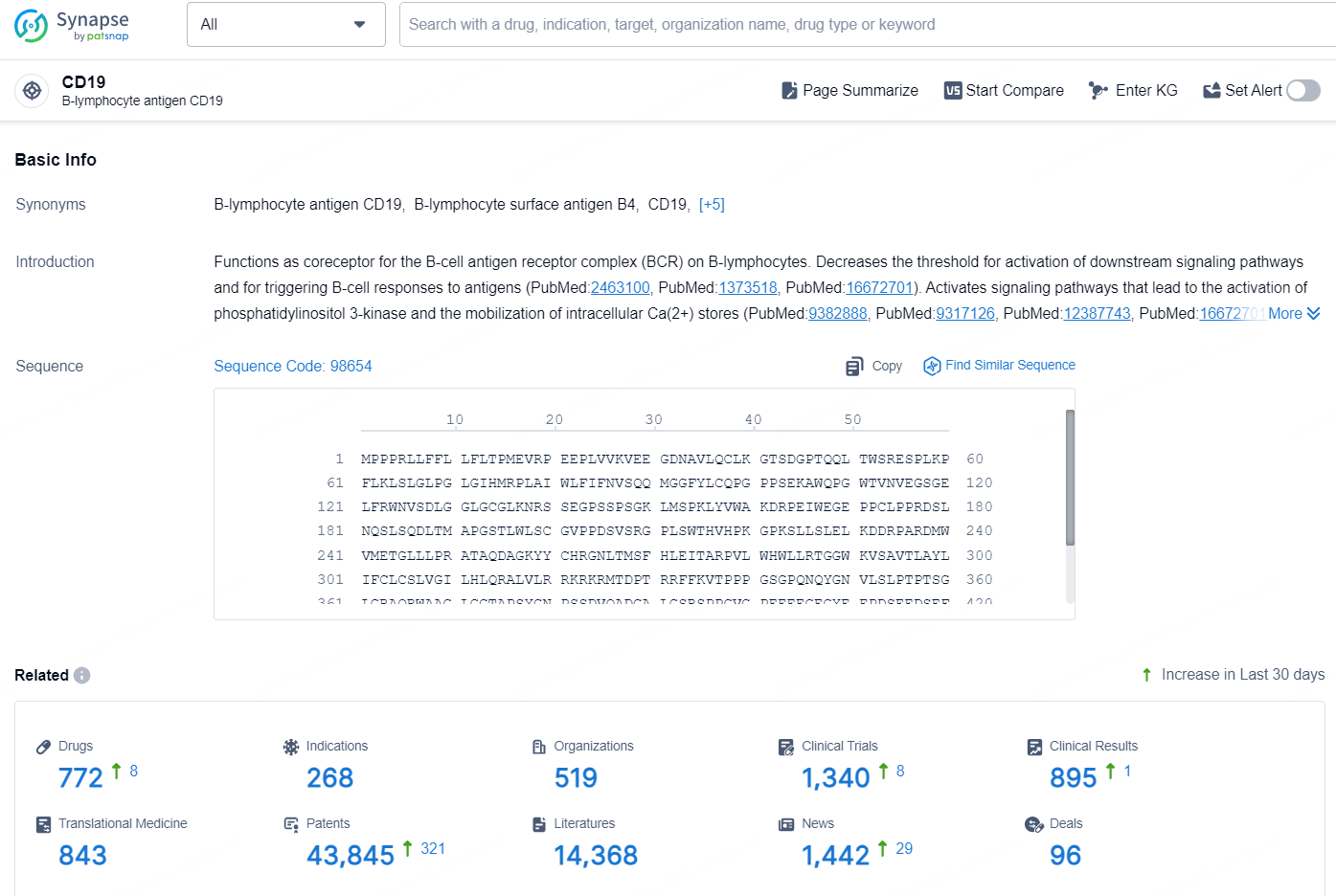
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 16, 2024, there are 772 investigational drugs for the CD19 targets, including 268 indications, 519 R&D institutions involved, with related clinical trials reaching 1340, and as many as 43845 patents.
Inebilizumab-cdon is a monoclonal antibody drug that targets CD19 and is used in the treatment of various therapeutic areas, including neoplasms, immune system diseases, infectious diseases, nervous system diseases, eye diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases.