An analysis of BNT-116's R&D progress and its clinical results presented at the 2023 SITC
BNT116 is an intravenously administered RNA-lipoplex therapeutic cancer vaccine comprising six RNAs each encoding a tumor-associated antigen (TAA) frequently expressed in non-small cell lung cancer (NSCLC). The preliminary results from patients with advanced unresectable or metastatic NSCLC (ECOG 0–2) receiving BNT116 monotherapy with optional addition of cemiplimab was reported in 2023 SITC Congress.
BNT-116's R&D Progress
BNT-116 is an RNA vaccine and therapeutic vaccine that is being developed by BioNTech SE. As an RNA vaccine, BNT-116 utilizes the genetic material of the virus or disease it is targeting to stimulate an immune response in the body. The drug is primarily focused on treating neoplasms and respiratory diseases. Its active indications include advanced lung non-small cell carcinoma and non-small cell lung cancer. 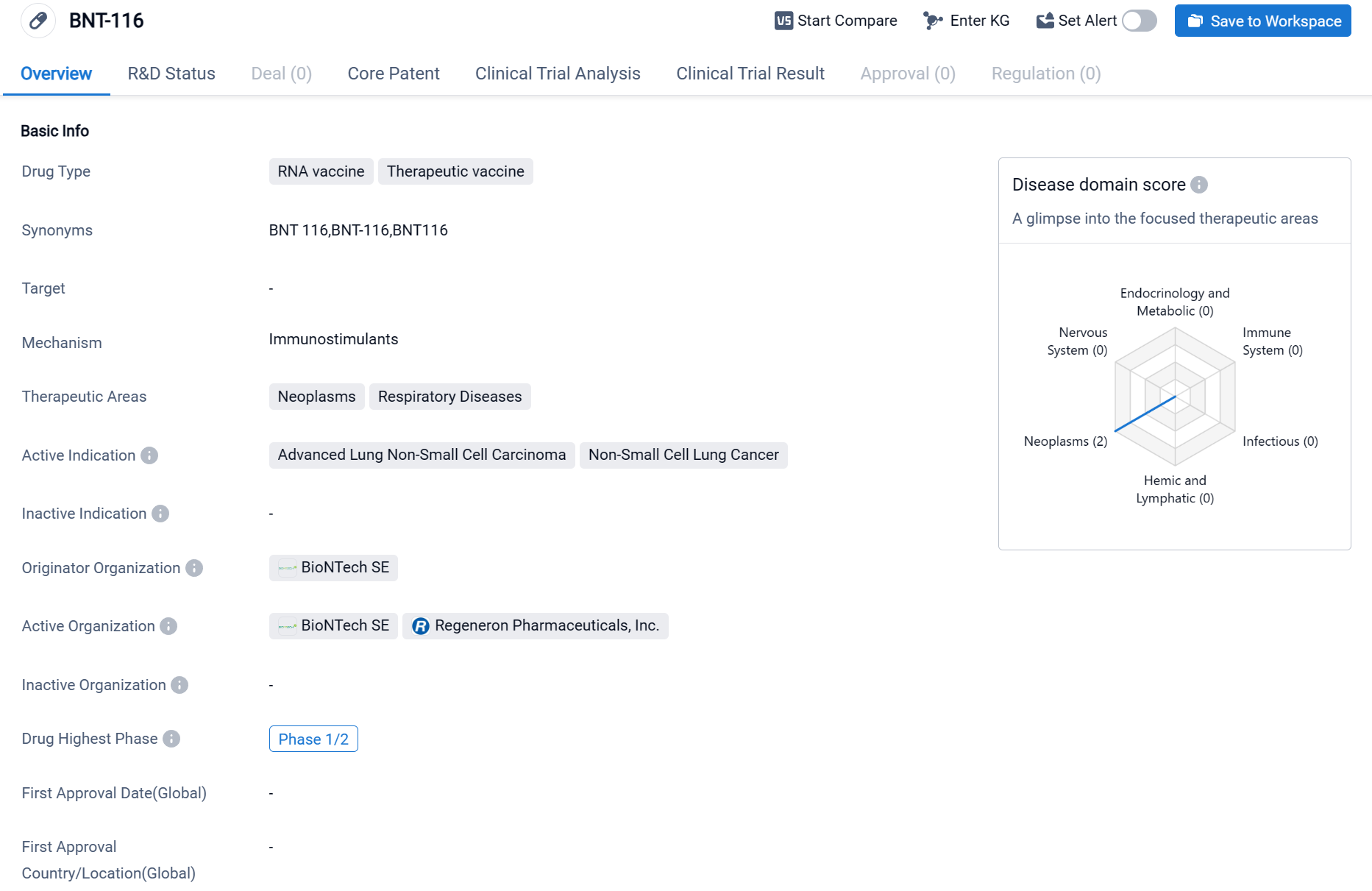
According to the Patsnap Synapse, the highest phase of development for BNT-116 is Phase 1/2, indicating that the drug has progressed to early-stage clinical trials. And the clinical trial areas for BNT-116 are primarily in the United States, Germany and Hungary. The key indication is Advanced Lung Non-Small Cell Carcinoma. 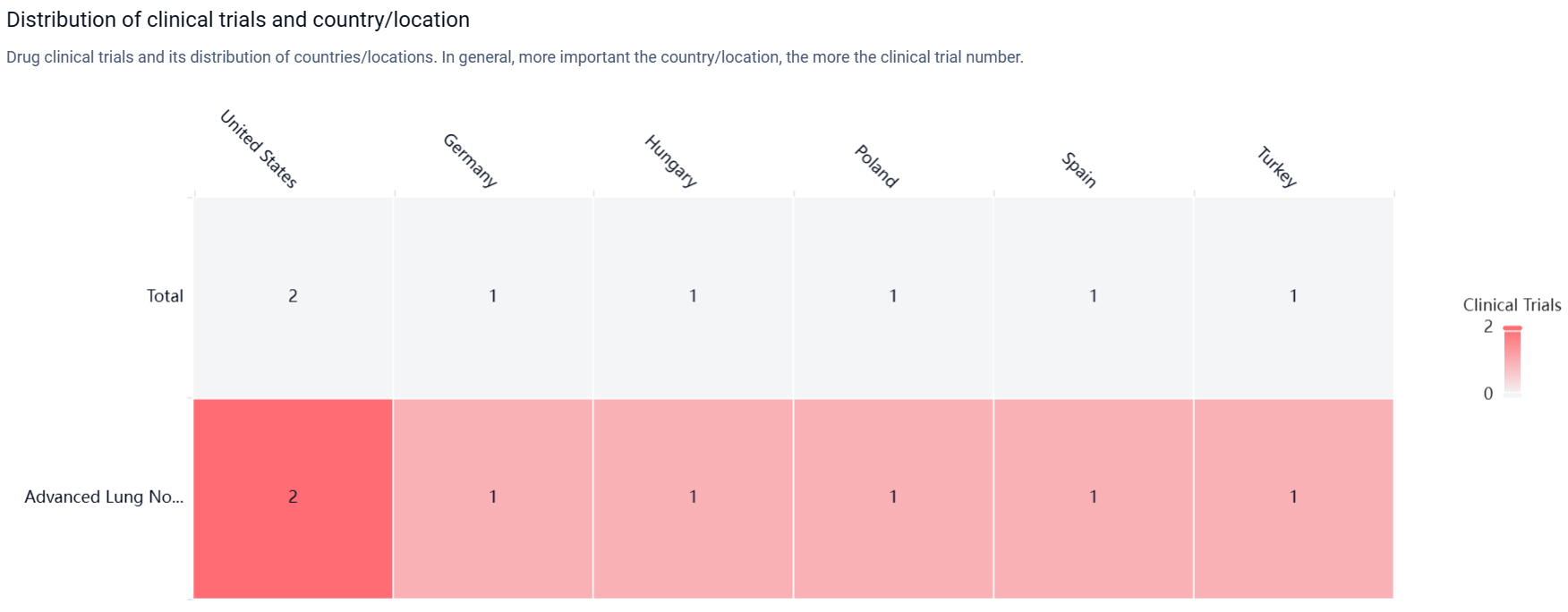
Detailed Clinical Result of BNT-116
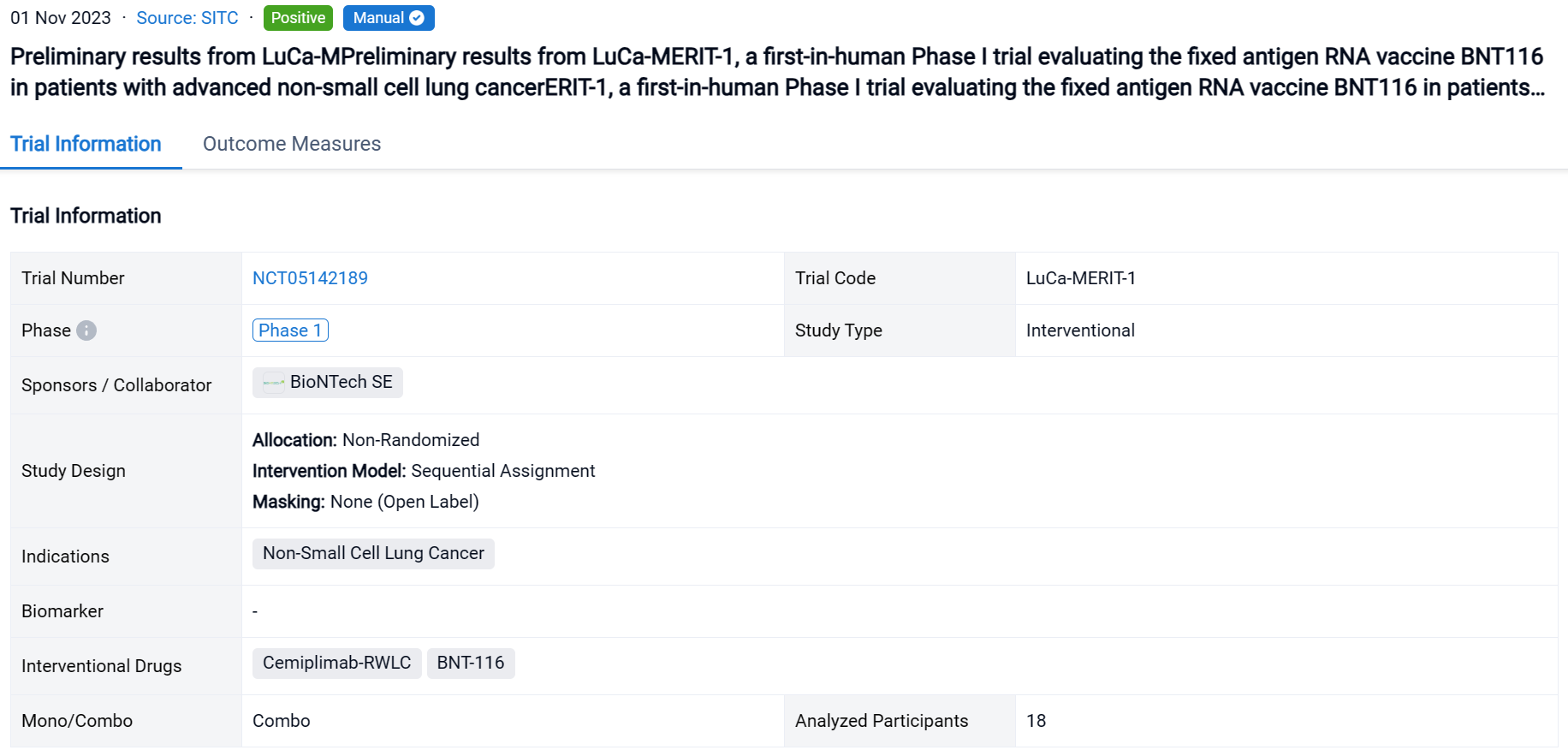
The non-randomized, sequential assignment, open-label clinical trial (NCT05142189) was aimed to confirm the dose of BNT116 alone or as combination therapy with cemiplimab, docetaxel, and/or carboplatin/paclitaxel (21-day cycles).
In this study, the first six BNT116 doses are administered once weekly during Cycles 1 and 2 and three-weekly from Cycle 3 onwards. The cohort reported here will confirm the dose of BNT116 as monotherapy. Cemiplimab may be added from cycle 3 if tumor samples exhibit PD-L1 expression (tumor proportion score ≥1%). Patients’ prior therapies must have included a PD-1/PD-L1 inhibitor, a platinum-based chemotherapy regimen as well as one other systemic therapy. The objectives are to determine safety (dose limiting toxicities [DLTs] in Cycle 1; treatment-emergent adverse events [TEAEs]), and clinical activity (RECIST v1.1). Tumor and blood samples will be used for biomarker analysis.

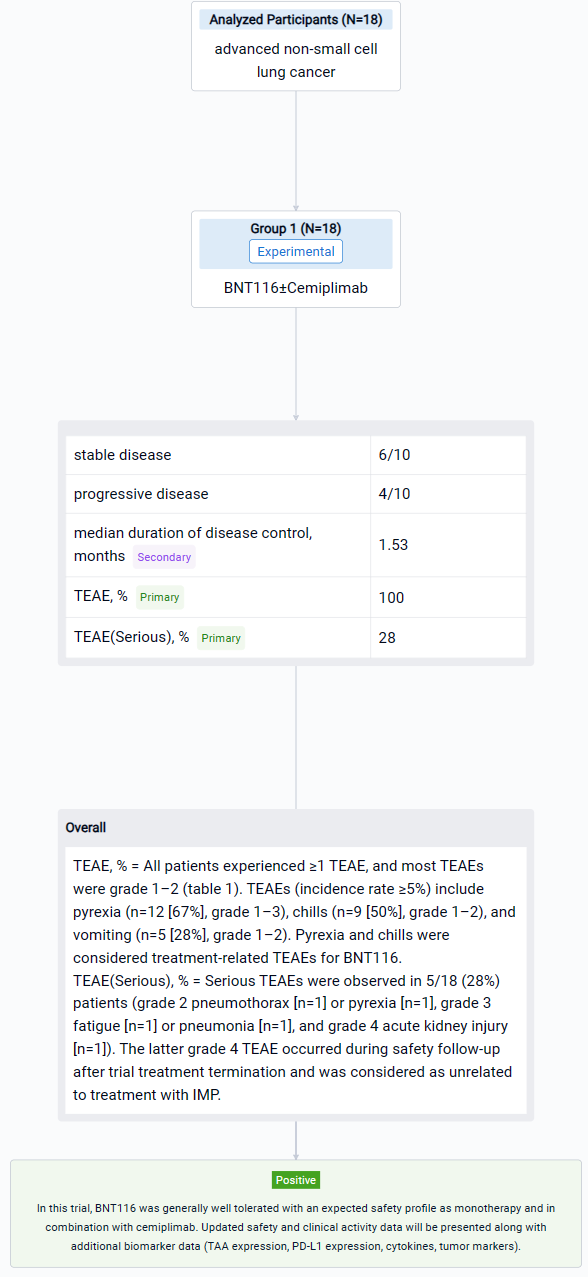
The result showed that as of 01 March 2023, 18 patients (median age 65 years) have received BNT116 in the reported cohort (n=13 monotherapy only; n=5 cemiplimab added after Cycle 3). Most (16/18 [89%]) had received ≥3 prior therapy lines. All patients experienced ≥1 TEAE, and most TEAEs were grade 1–2 (table 1). TEAEs (incidence rate ≥5%) include pyrexia (n=12 [67%], grade 1–3), chills (n=9 [50%], grade 1–2), and vomiting (n=5 [28%], grade 1–2). Pyrexia and chills were considered treatment-related TEAEs for BNT116. Serious TEAEs were observed in 5/18 (28%) patients (grade 2 pneumothorax [n=1] or pyrexia [n=1], grade 3 fatigue [n=1] or pneumonia [n=1], and grade 4 acute kidney injury [n=1]). The latter grade 4 TEAE occurred during safety follow-up after trial treatment termination and was considered as unrelated to treatment with IMP. No DLTs or deaths under treatment were observed. Ten of 18 patients were evaluable for clinical activity (n=5 monotherapy only; n=5 cemiplimab added after Cycle 3). Of these, 6/10 had stable disease and 4/10 had progressive disease. The median duration of disease control was 1.53 months.
It can be concluded that in this trial, BNT116 was generally well tolerated with an expected safety profile as monotherapy and in combination with cemiplimab. Updated safety and clinical activity data will be presented along with additional biomarker data (TAA expression, PD-L1 expression, cytokines, tumor markers).
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
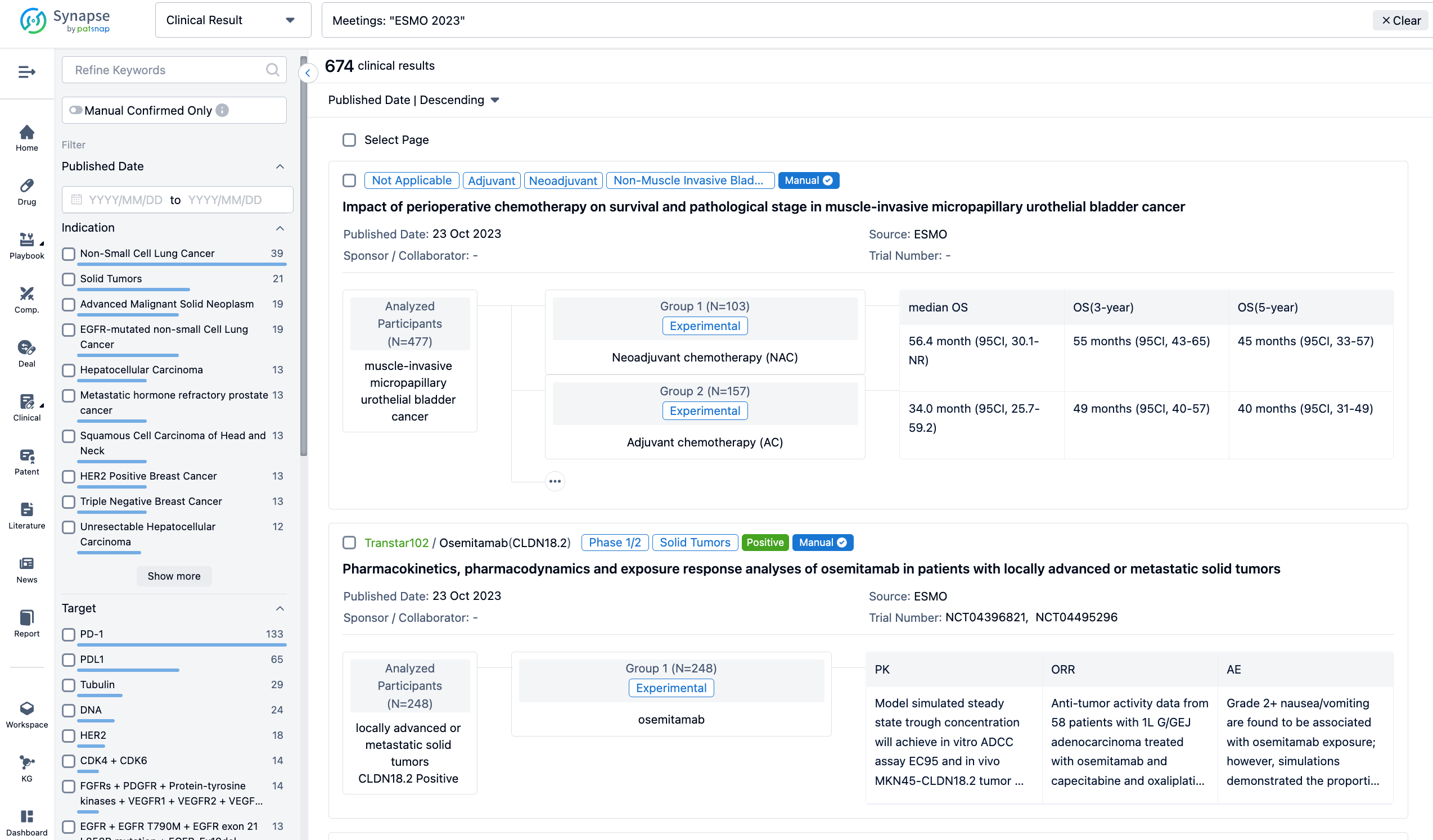
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

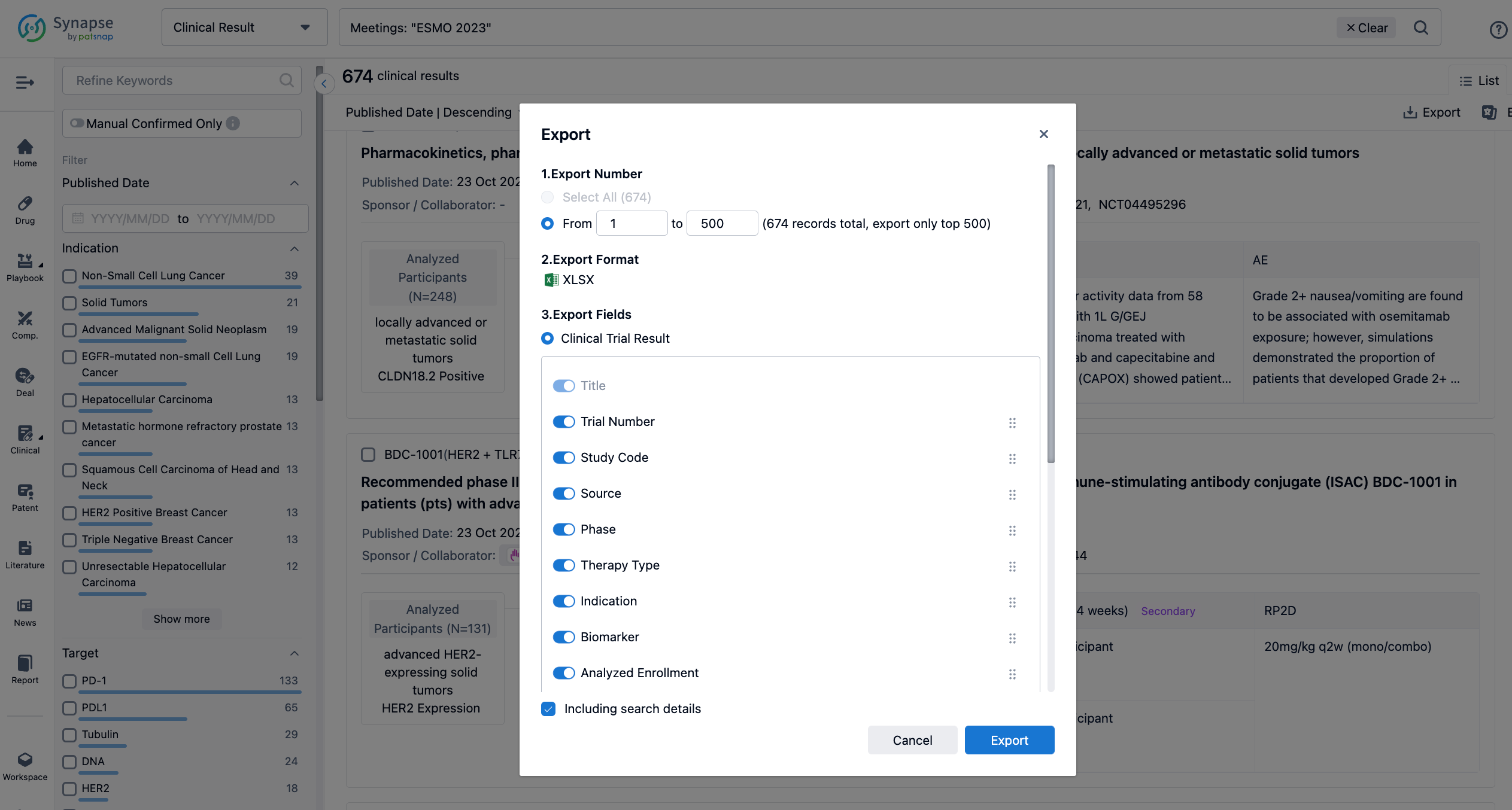
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!