Ionetix will supply Bayer with Actinium-225 (Ac-225), a therapeutic radioisotope
Ionetix Corporation has publicized that it has entered into a supply contract for the therapeutic radioisotope actinium-225 (Ac-225) with Bayer. According to the contract, Ionetix will be responsible for supplying Bayer with a pure, non-carrier added form of Ac-225.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Ac-225, an alpha-emitter, can provide cancer-killing radiation directly to tumors when combined with a tumor-targeting molecule. This eliminates cancer cells while leaving the healthy tissue surrounding the tumor untouched. Utilizing targeted radionuclide therapies for precise cancer targeting shows significant potential as a novel approach to cancer treatment. Nonetheless, Ac-225's global availability is quite restricted with only a handful of production resources available at present, signaling a pressing need for introducing more Ac-225 production sources that can efficiently scale to support the growth of these novel cancer treatments.
David Eve, VP of Medical Affairs for Ionetix Corporation, conveyed that their company foresaw the rising need and acted proactively by investing in creating a solution for Ac-225's mass production by leveraging their proficiency in target technology and cyclotron accelerator. Ionetix, based in Lansing, Michigan, recently launched an isotope production facility which is entirely committed to the production of alpha-emitters.
At the facility, the first cyclotron was up and running earlier this year, with plans for a second cyclotron to be installed by 2024. Both the cyclotrons will be dedicated to alpha-emitter production, bringing in both onsite redundancy and an increased production capacity. By early 2024, the facility will commence the distribution of n.c.a. Ac-225 of GMP grade.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
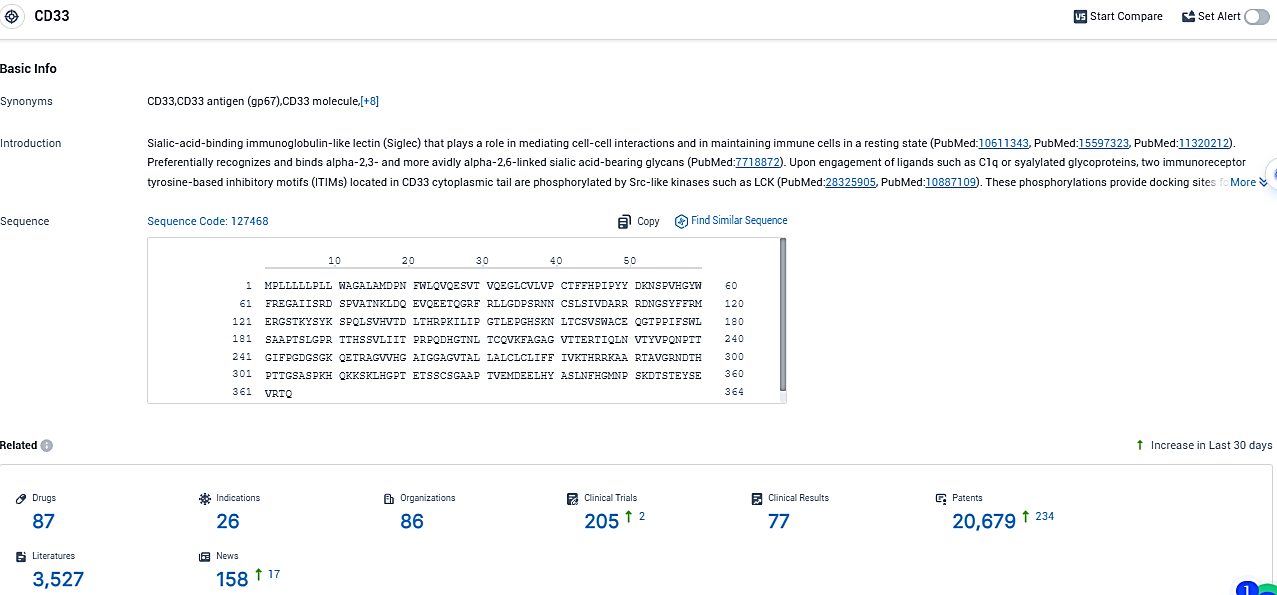
According to the data provided by the Synapse Database, As of November 21, 2023, there are 87 investigational drugs for the CD33 target, including 26 indications, 86 R&D institutions involved, with related clinical trials reaching 205, and as many as 20679 patents.
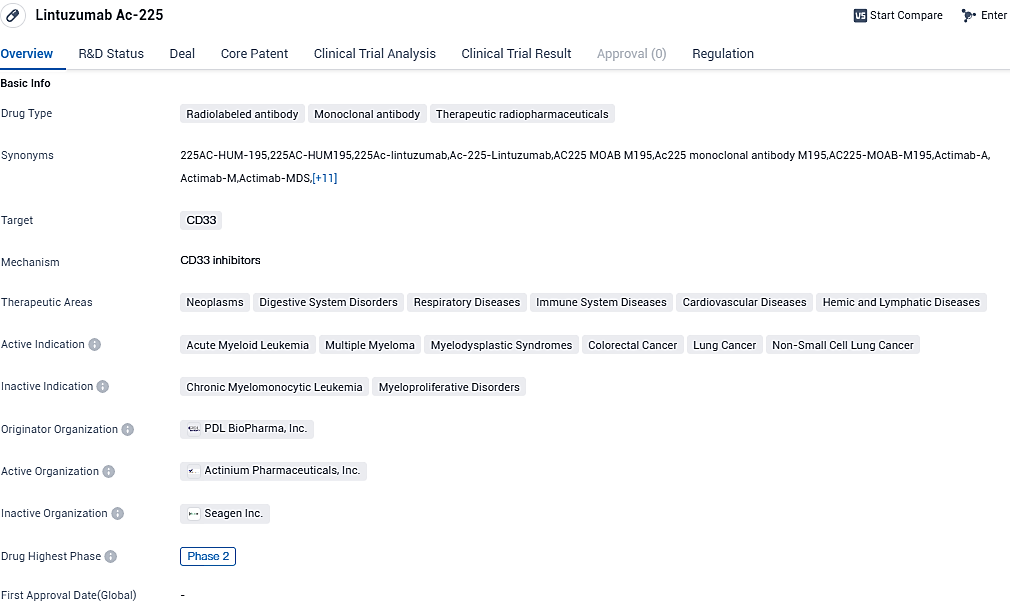
Lintuzumab Ac-225 is a radiolabeled antibody and monoclonal antibody that targets CD33. It has shown potential in the treatment of various neoplasms and other diseases affecting different organ systems.