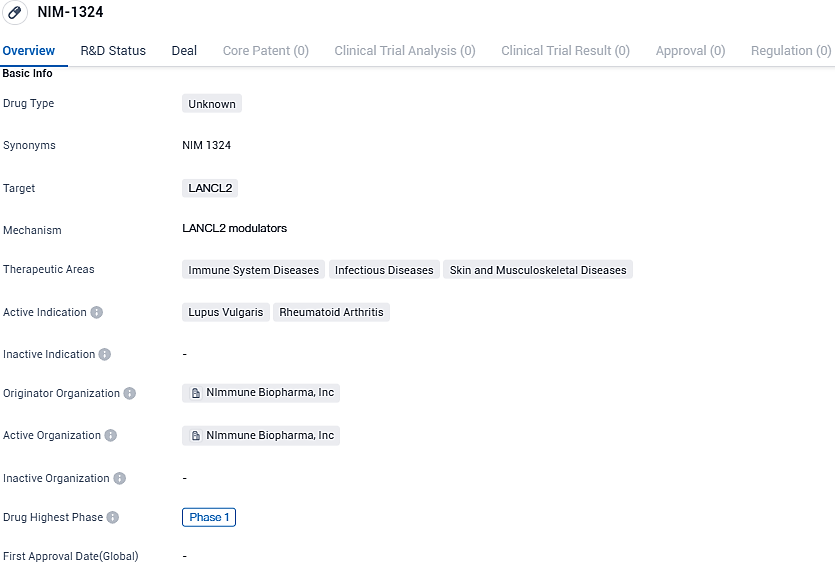
NImmune Biopharma released promising Phase 2 results for their lupus drug NIM-1324 at the 2023 ACR Convergence
NImmune Biopharma, a privately held, advanced clinical-stage biopharma entity specializing in precision immunology, has declared the presentation of a report highlighting the successful Phase 1 clinical trial results of NIM-1324, an orally administered, once-daily, systemically circulated LANCL2 agonist therapy intended for treating rheumatic diseases, at the yearly assembly of the American College of Rheumatology.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Systemic lupus erythematosus still has substantial unaddressed healthcare requirements. The preliminary human study of our proprietary NIM-1324 for SLE successfully met all primary and secondary objectives concerning safety and tolerability.
Coupled with data from a corresponding blood-based precision medicine diagnostic, we are greatly hopeful about the potential of NIM-1324 to provide the pioneering precision therapy for SLE, introducing a unique mode of action without dose-dependent toxicities," conveyed Dr. Josep Bassaganya-Riera, NImmune’s Founder and Chief Executive Officer. Dr. Josep Bassaganya-Riera furthered, “Upon our latest declaration of promising Phase 2 outcomes for Omilancor and the clinical affirmation of the LANCL2 pathway in Ulcerative Colitis and Crohn's disease, we're excited to disclose the progression of our second therapeutic contender into Phase 2 clinical trials for Lupus, accompanied by imminent opportunities in other autoimmune diseases.
The initial patient for the forthcoming Phase 2 trial of NIM-1324 in SLE is expected in 2024, with primary outcome data projected by 2025.” The clinical outcomes of NIM-1324 disclosed at ACR'23 utilised the TITAN-X platform, an Artificial Intelligence-based discovery and development platform that has directed the cultivation of NImmune's LANCL assortment of therapeutics.
This includes NIM-1324 and omilancor, both oral, daily agonistic therapeutics that establish and activate the LANCL2 pathway. Omilancor is intentionally constructed for targeted discharge in the gastrointestinal tract for IBD symptoms, and NIM-1324 is engineered to be extensively systematically disseminated for ailments including lupus and rheumatoid arthritis.
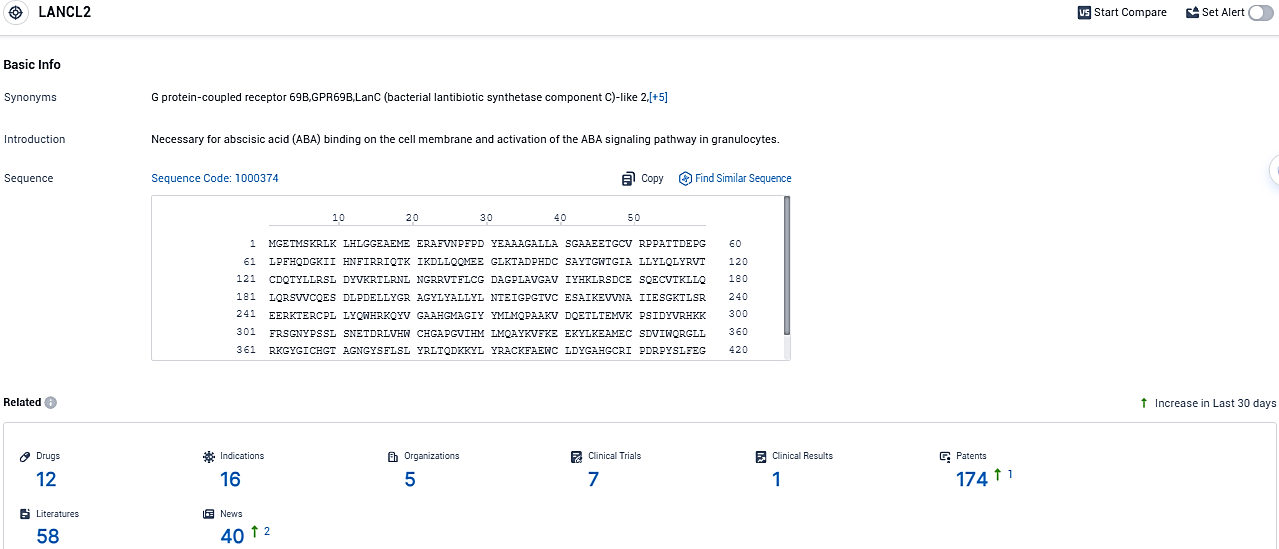
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 19, 2023, there are 12 investigational drugs for the LANCL2 target, including 16 indications, 5 R&D institutions involved, with related clinical trials reaching 7, and as many as 174 patents.
NIM-1324 is an oral, systemically distributed, small-molecule therapeutic candidate which activates LANCL2, a surface membrane-associated receptor that is responsible for modulating key cellular and molecular changes tied to autoimmune diseases. NIM-1324 target U.S. market size is expected to be valued at $226.0 billion 2021-2030, of which a peak annual market size of $23.1 billion is expected to occur in 2030.