An analysis of ENB-003's R&D progress and its clinical results presented at the 2023 SITC
The 2023 SITC Congress kicked off with a groundbreaking report on the latest clinical results of ENB-003, establishing a solid foundation for future research.
ENB-003's R&D Progress
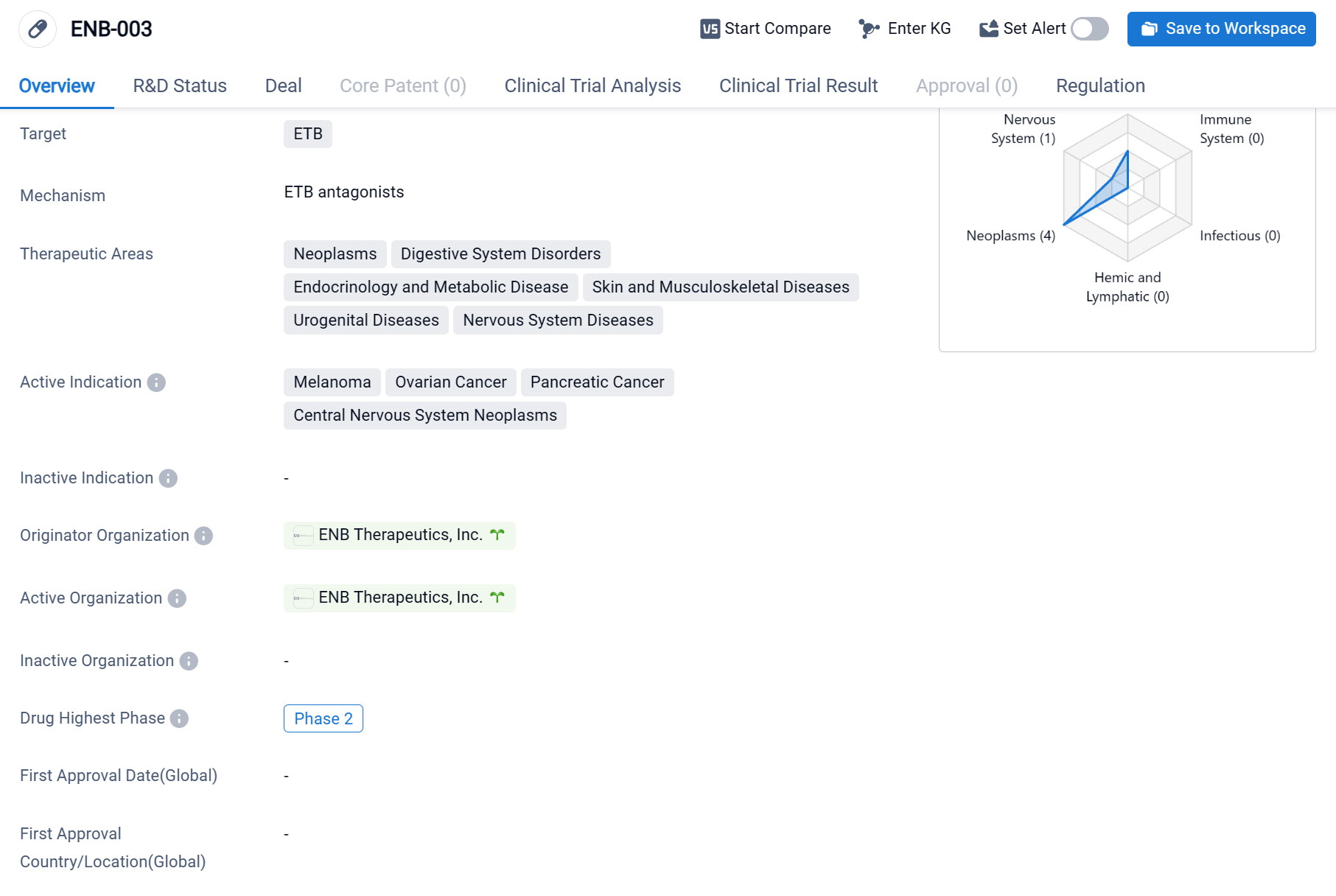
ENB-003 is a small molecule drug developed by ENB Therapeutics, Inc. The drug targets the ETB, or endothelin B receptor, which is involved in various physiological processes. In terms of therapeutic areas, ENB-003 shows potential in treating a wide range of diseases. These include neoplasms (abnormal growth of cells), digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, and nervous system diseases. This broad spectrum of therapeutic areas sggests that ENB-003 may have a versatile mechanism of action or multiple targets.
According to the Patsnap Synapse, ENB-003 is currently in Phase 2 of clinical trials. And the clinical trial areas for ENB-003 are primarily in the United States and Australia. The key indication is Ovarian Cancer. 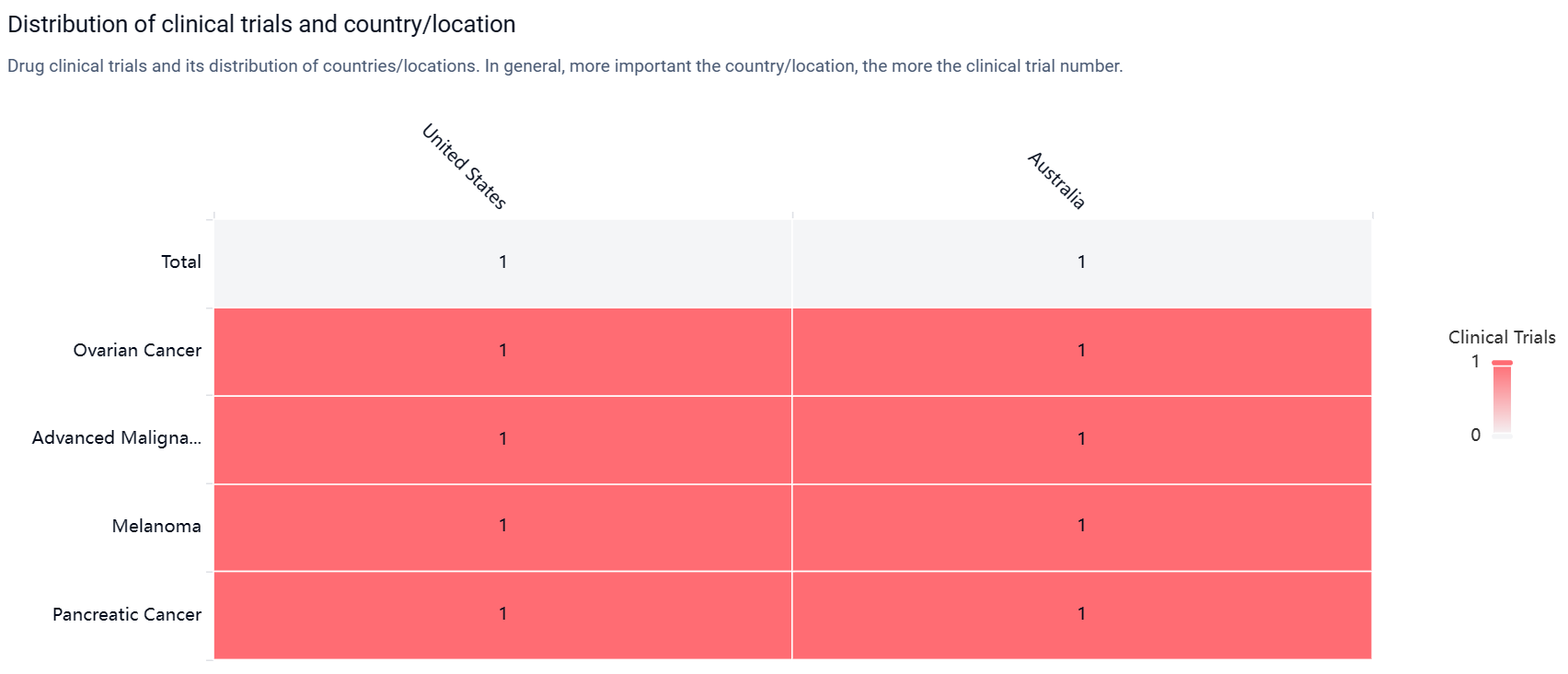
Detailed Clinical Result of ENB-003
The non-randomized, single group assignment, open-labeled clinical trial (NCT04205227) was aimed to investigate the safety and efficacy of ENB-003 in combination with pembrolizumab in solid tumors refractory to standard of care therapies including ovarian cancer.
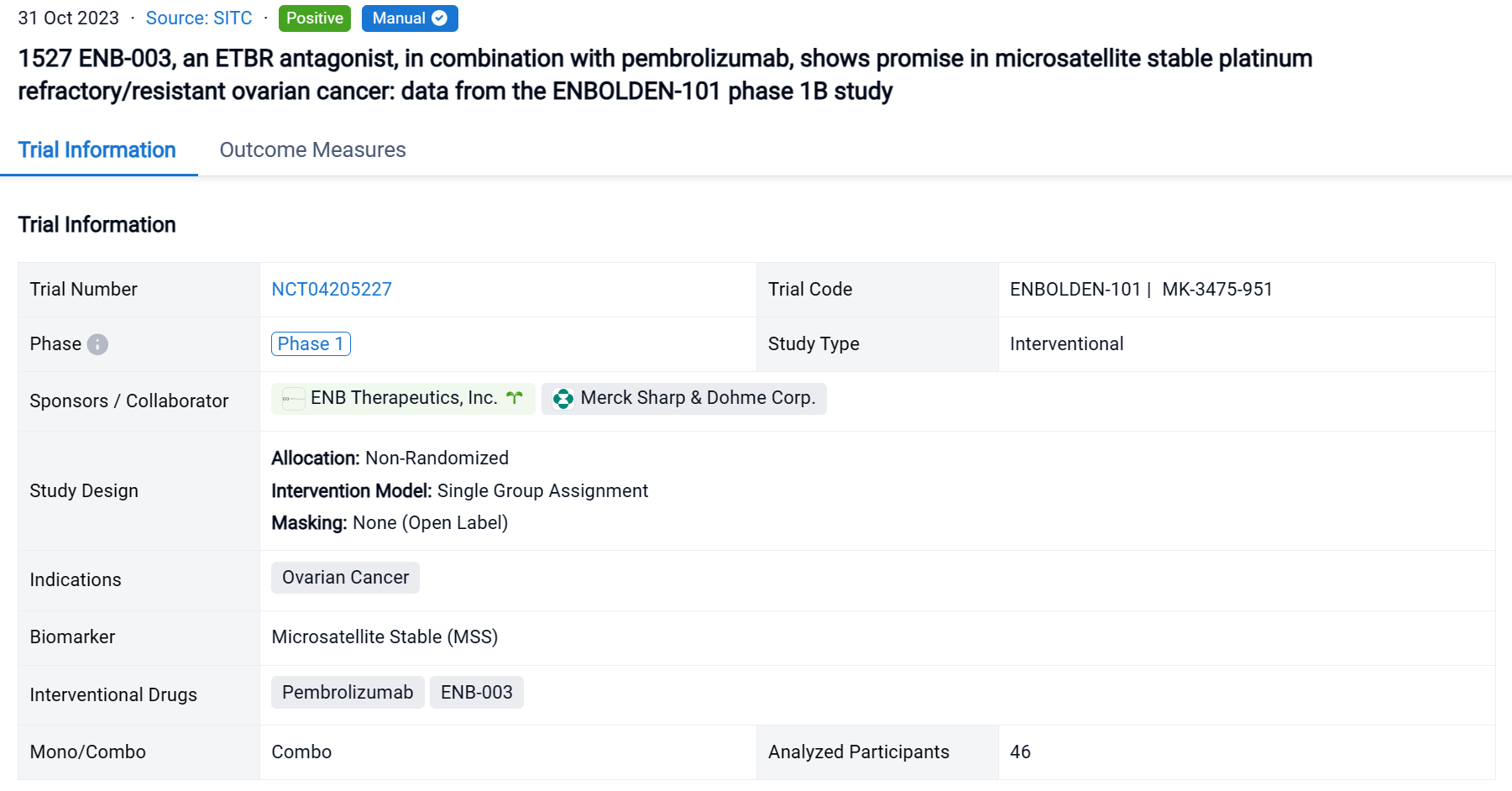
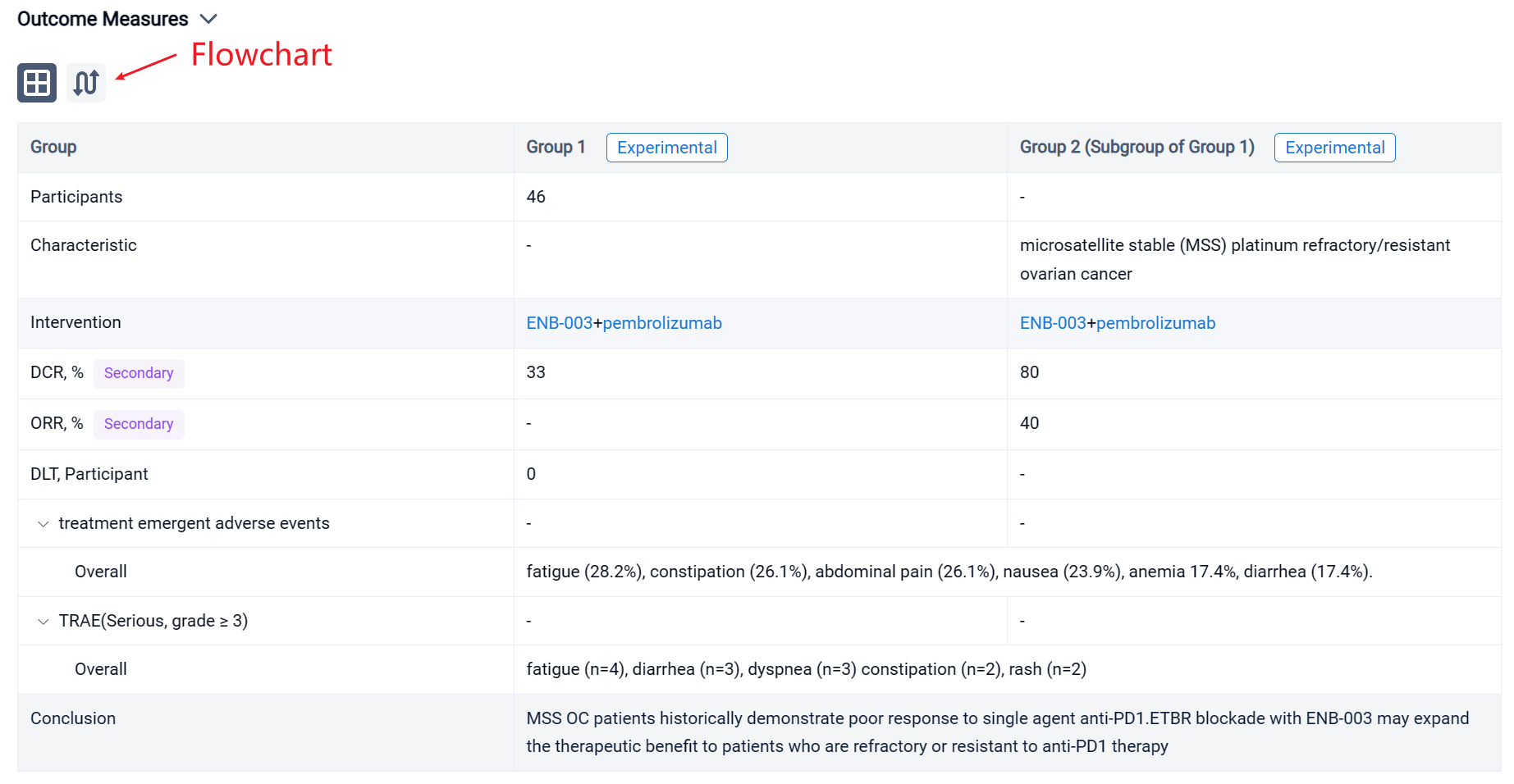
In this study, the Part 1 3+3 dose escalation enrolled 46 subjects and included 6 escalating doses of ENB-003 (ranging from 150ug-2000ug) in combination with a fixed dose of pembrolizumab (200mg). Pembrolizumab was administered once every 21-day cycle. ENB-003 was administered IV as a single agent during a 1-week monotherapy run-in, followed by combination therapy with pembrolizumab. ENB-003 was administered 3x per week for a total of 6 doses in odd numbered cycles for the first 5 cohorts and administered every cycle for the last and 6th cohort. The primary objective of Part 1 was to assess safety and tolerability, secondary objectives included PFS, OS and ORR by RECIST 1.1, iRECIST. Exploratory objectives are to examine biomarkers/pharmacodynamics.

The result showed that no DLTs were observed across 6 dosing cohorts. The most common treatment emergent adverse events irrespective of grade or causality included fatigue (28.2%), constipation (26.1%), abdominal pain (26.1%), nausea (23.9%), anemia 17.4%, diarrhea (17.4%). Serious adverse events, grade 3 and above considered possibly related to pembrolizumab and/or ENB-003 include fatigue (n=4), diarrhea (n=3), dyspnea (n=3) constipation (n=2), rash (n=2). The DCR across all cohorts irrespective of ETBR status was 33% (2 PR, 8 SD, 20 PD). For microsatellite stable (MSS) platinum refractory/resistant ovarian cancer (OC) there was a 40% ORR and an 80% DCR across all cohorts (2 PR, 2 SD, 1 PD) with a trend for durable responses at higher doses of ENB-003. Responses included a 95% PR of 12-month duration in a primary platinum refractory MSS OC patient.
It can be concluded that MSS OC patients historically demonstrate poor response to single agent anti-PD1.ETBR blockade with ENB-003 may expand the therapeutic benefit to patients who are refractory or resistant to anti-PD1 therapy.
How to Easily View the Clinical Results Using Synapse Database?
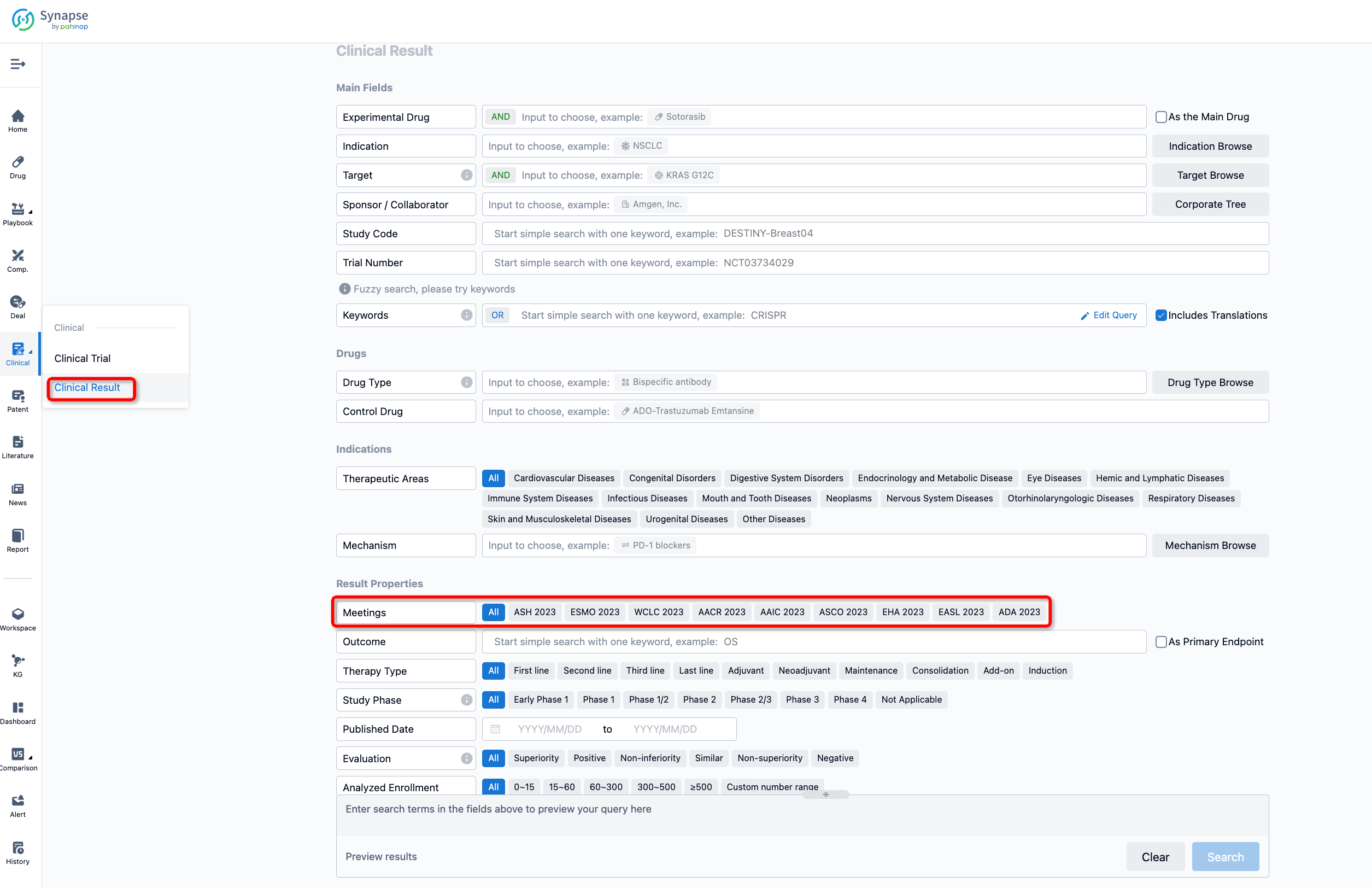
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
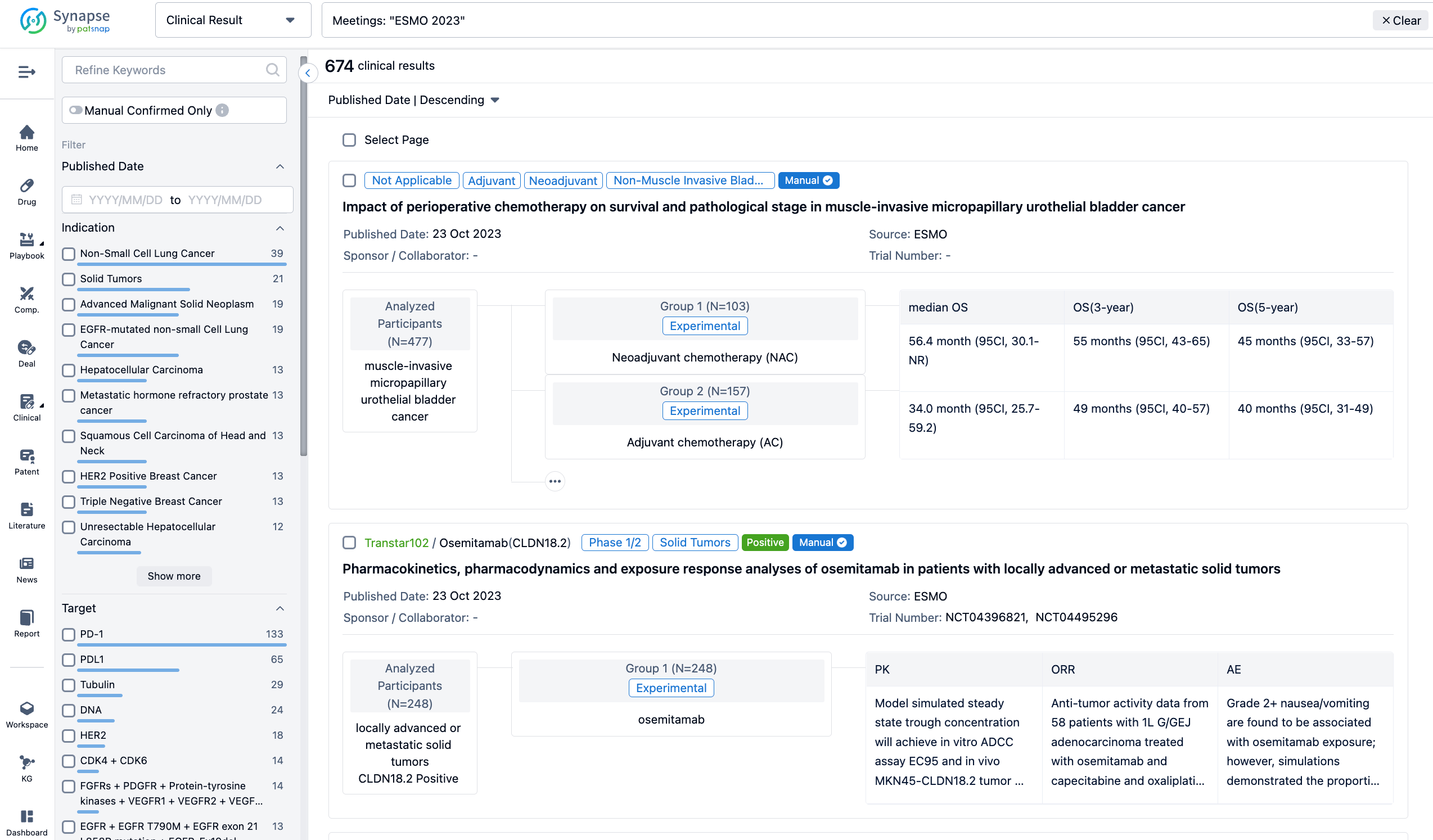
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
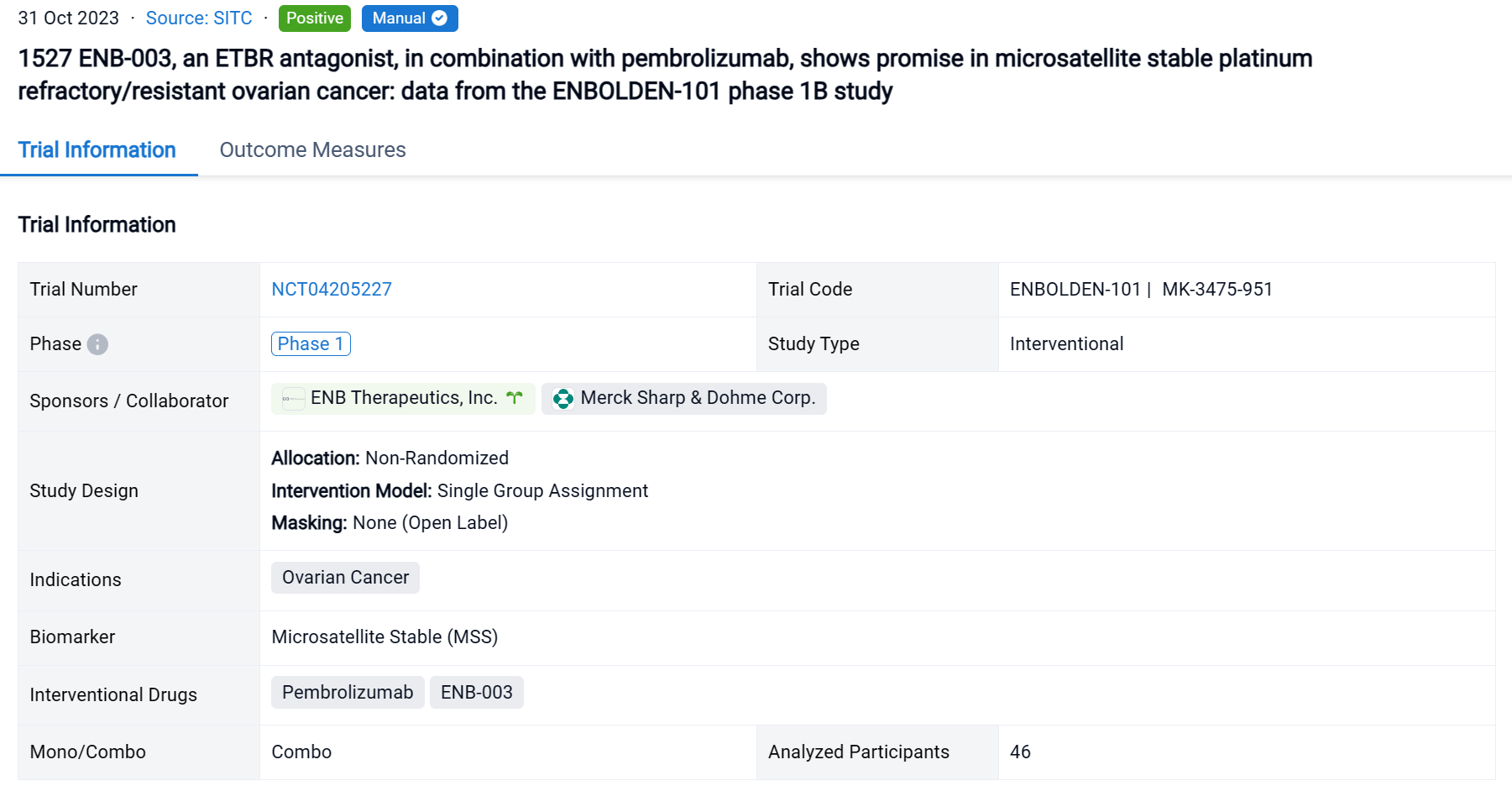
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

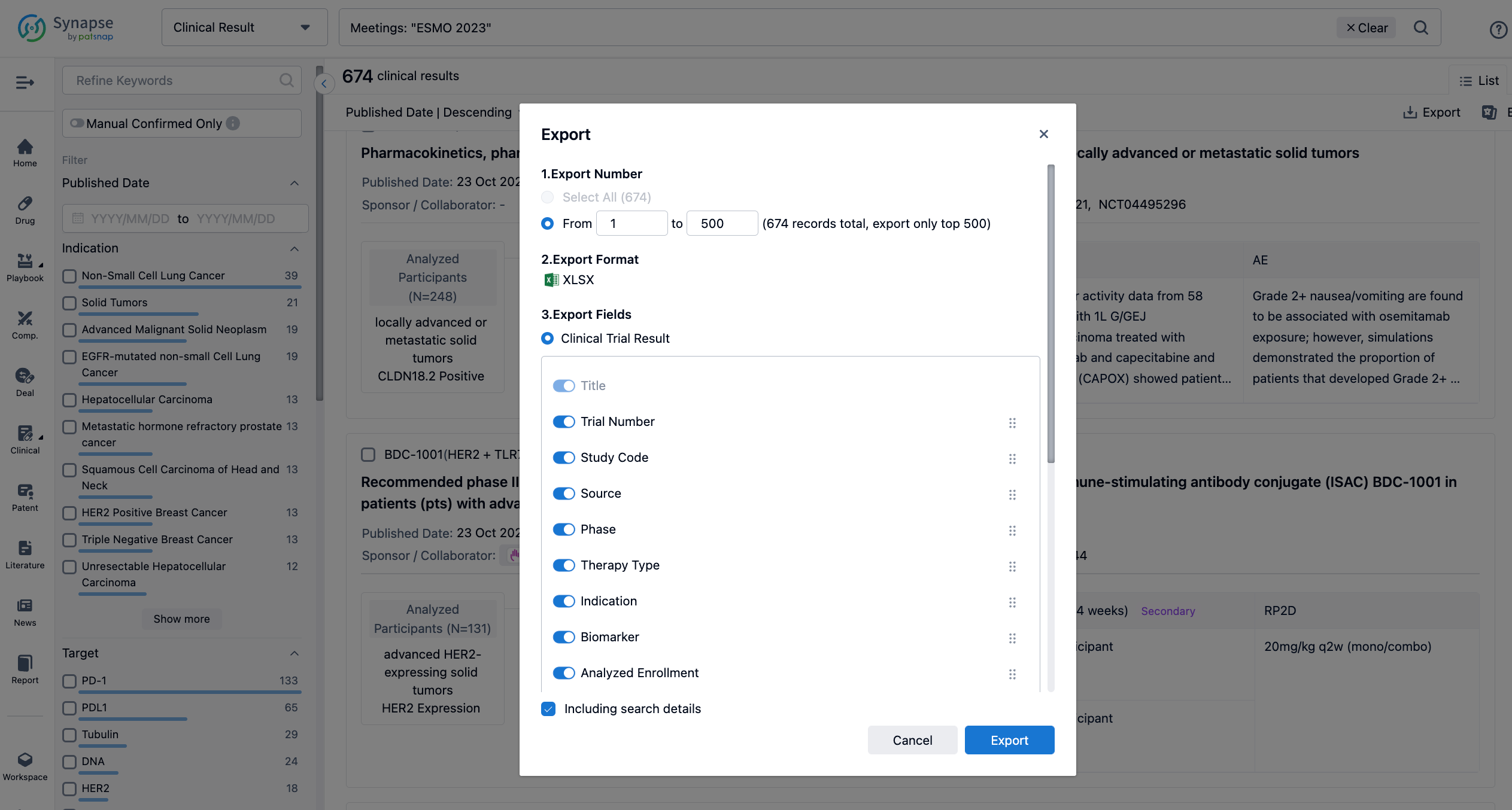
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!