Latest updates on PACIFIC-2 Phase III trial studying Imfinzi with chemo and radiation for inoperable Stage III non-small cell lung cancer
The Phase III PACIFIC-2 study on Imfinzi (durvalumab) combined with chemoradiotherapy failed to meet the primary endpoint of PFS significance when compared to CRT alone, in treating patients diagnosed with unresectable, Stage III NSCLC.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Imfinzi, when administered sequentially following platinum-based CRT, sets the global standard care for handling non-operable stage III NSCLC, which is confirmed by the outcomes from the PACIFIC Phase III study. That said, the PACIFIC-2 study was rolled out to scrutinize the concurrent application of Imfinzi with CRT, targeted at patients who progress or halt treatment within their CRT phase thereby making them unfit for the PACIFIC regime.
An initial assessment of the safety and tolerance of Imfinzi and CRT in this patient group pointed out that the profiles tallied up with those generally recognized for these therapies; yet, it also exposed an augmented incidence of infection during the period of simultaneous treatment in the test group.
Jeffrey D. Bradley, MD, the Deputy Chair of Proton Therapy & Technological Advancement at Penn Medicine, Philadelphia, and chief investigator for the study stated: "Although the PACIFIC-2 study does not display the expected results, the PACIFIC treatment method continues to be the primary treatment for patients with non-operable, Stage III NSCLC. We as a fraternity, plan to use these results as learning for propelling future research.”
Susan Galbraith, who is the Executive Vice President of Oncology R&D at AstraZeneca, voiced: "Despite the results not meeting statistical relevance, we plan to use the learnings from this study to continuously strive towards enhancing the patient outcomes by widening the benefits of immunotherapy across different lung cancer treatment settings."
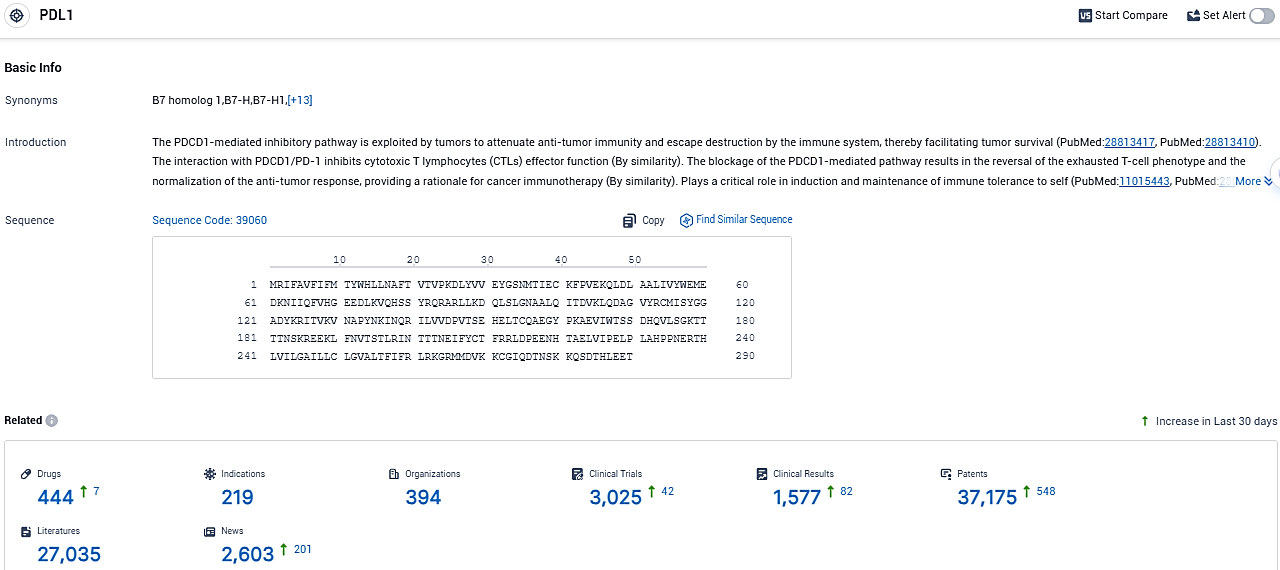
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 21, 2023, there are 444 investigational drugs for the PDL1 target, including 219 indications, 394 R&D institutions involved, with related clinical trials reaching 3025, and as many as 37175 patents.
Imfinzi (durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumour's immune-evading tactics and releasing the inhibition of immune responses. Imfinzi is also approved in the US, EU, Japan, China and many other countries around the world for the treatment of extensive-stage SCLC based on the CASPIAN Phase III trial.