MDNA-11: A Quick Look at Its R&D Progress and Clinical Results from the 2023 SITC
On 31 Oct 2023, the latest clinical result of interim PK/PD, safety and efficacy data of monotherapy dose escalation of a phase 1/2 study with MDNA11 in patients with advanced solid tumors was reported at the 2023 SITC Congress, demenstrating its potential efficacy and paving the way for future research.
MDNA-11's R&D Progress
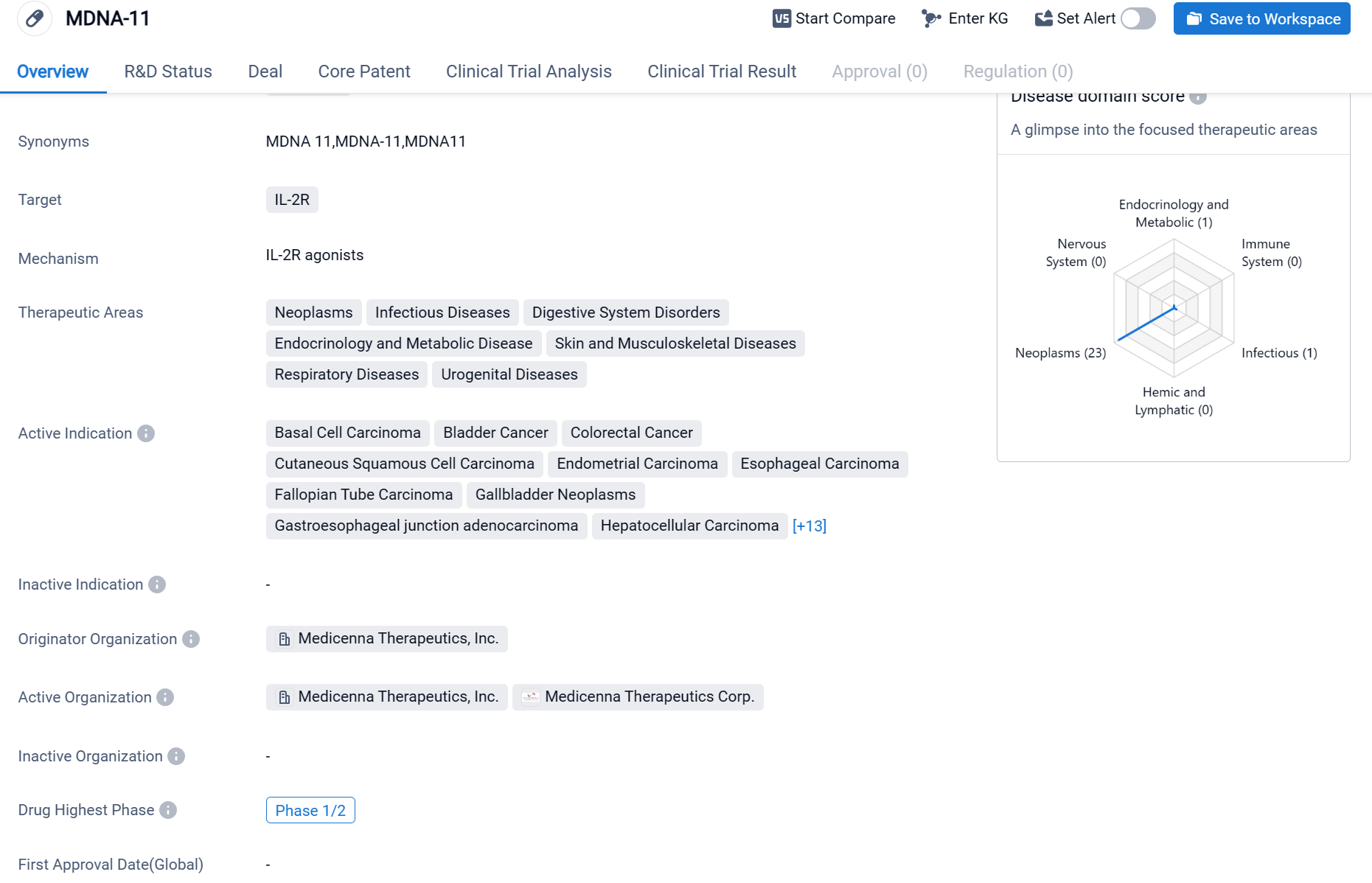
MDNA-11 is a cytokine drug that targets IL-2R, which has shown potential in treating a wide range of therapeutic areas including neoplasms, infectious diseases, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, respiratory diseases, and urogenital diseases.
According to the Patsnap Synapse, MDNA-11 is currently in the highest phase of clinical development, Phase 1/2, globally. And the clinical trial areas for MDNA-11 are primarily in the United States, Canada and Australia. The key indication is Microsatellite instability-high colorectal cancer. 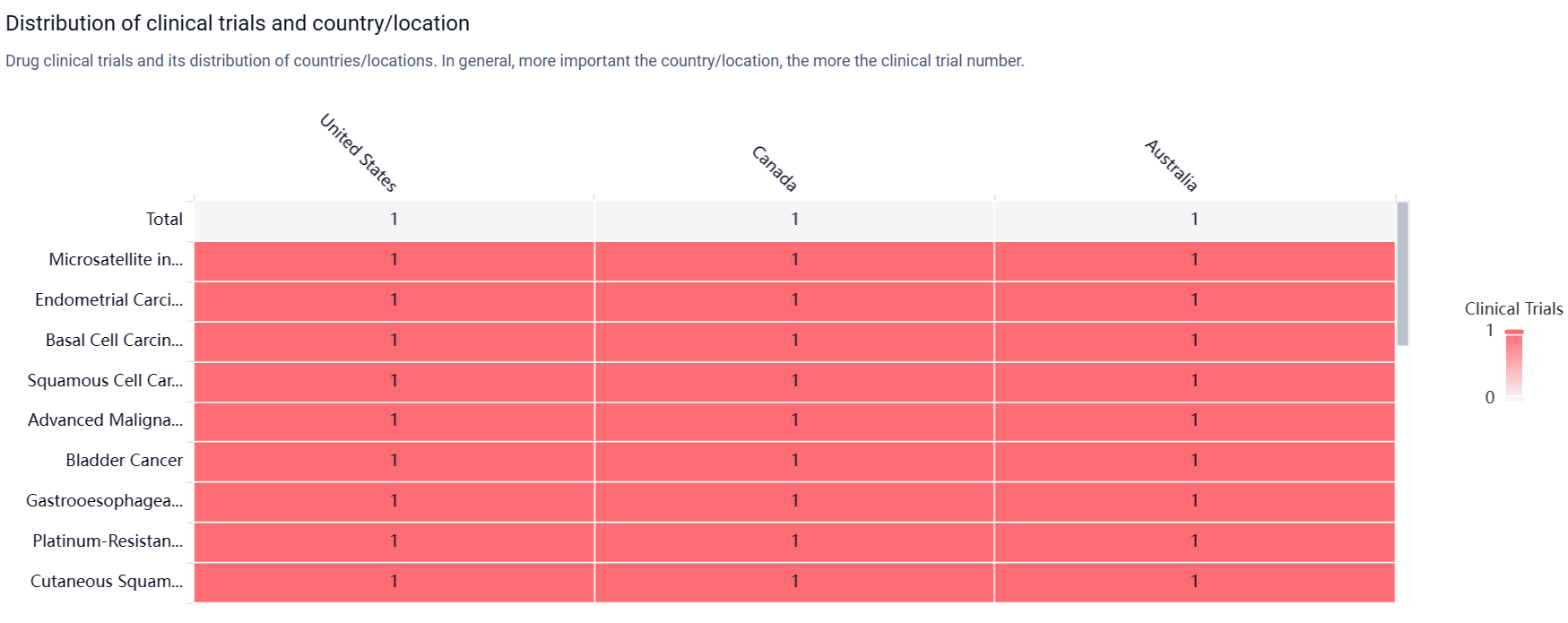
Detailed Clinical Result of MDNA-11
The sequential assignment, open-labeled clinical trial (NCT05086692) used a modified 3+3 design to determine the recommended dose for expansion (RDE).
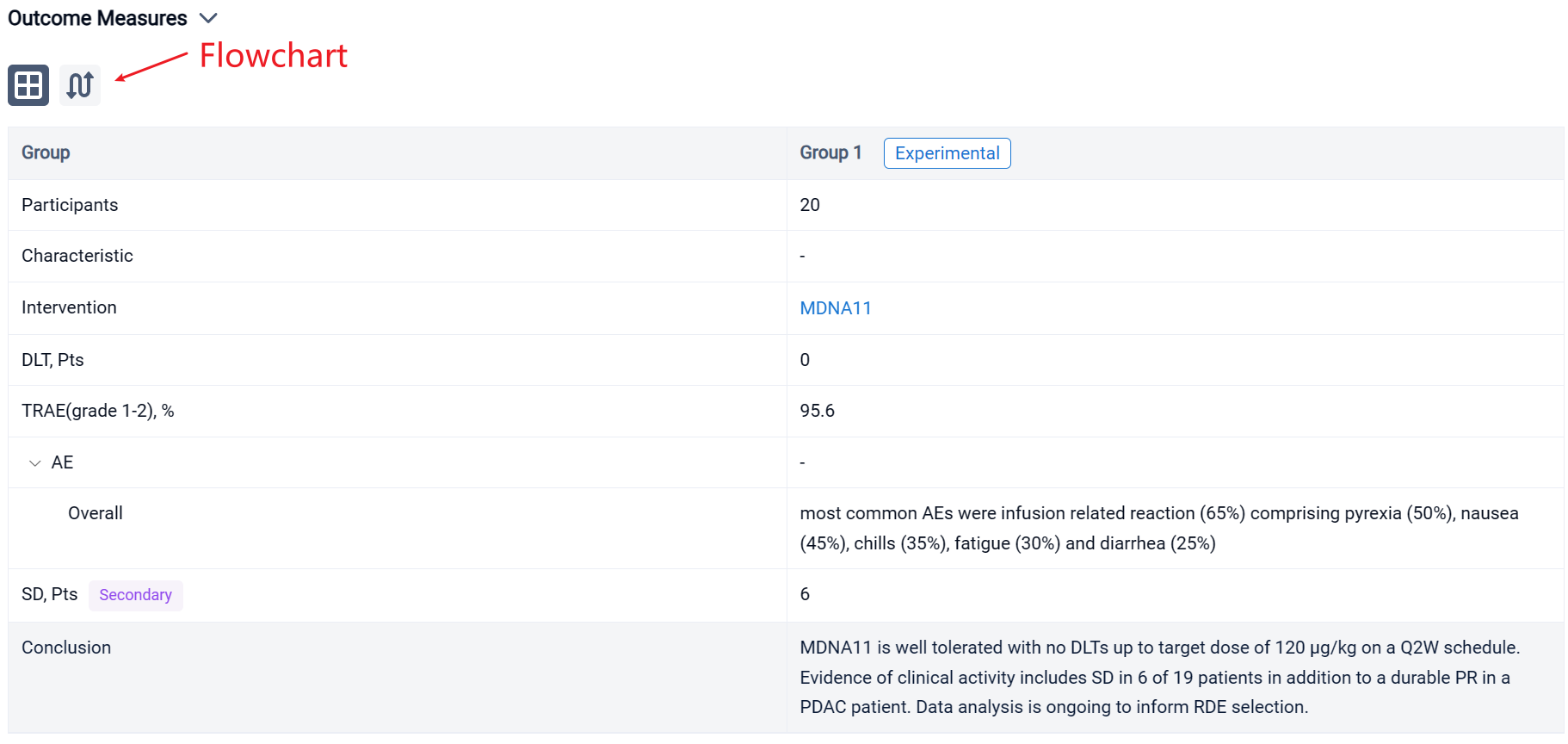
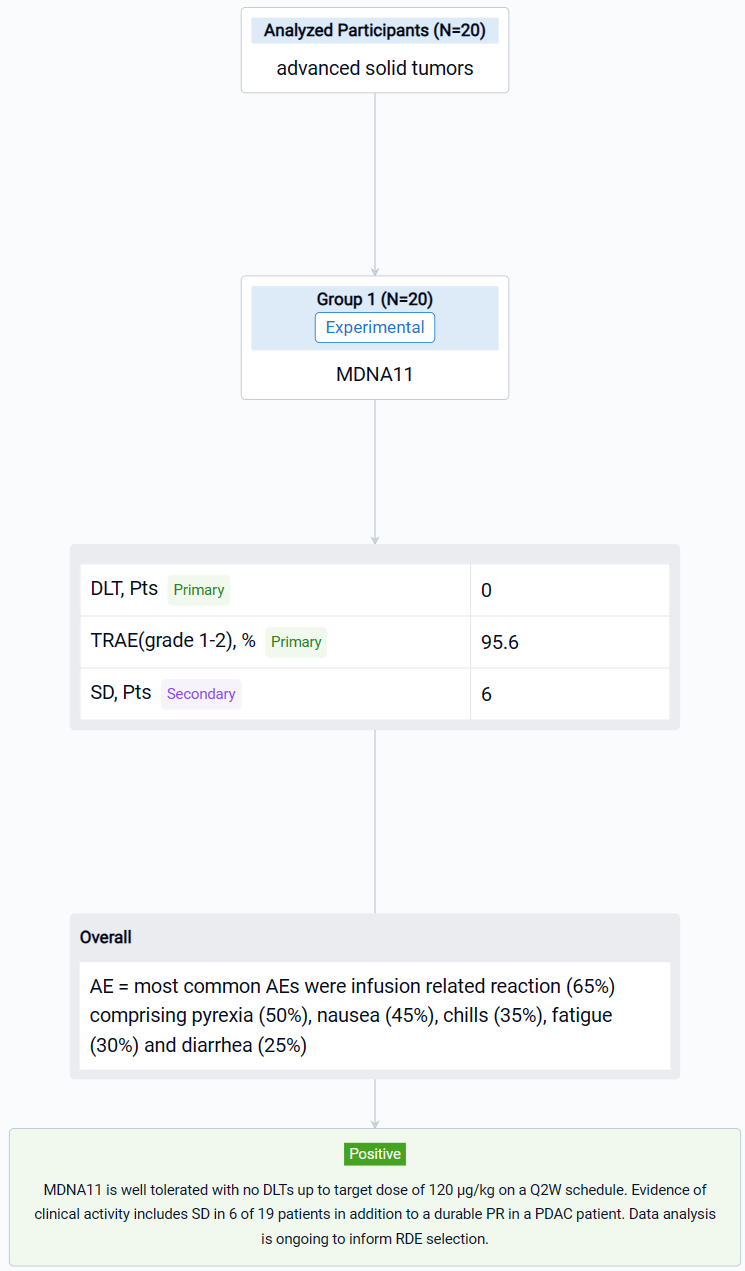
In this study, patients received a fixed dose of 3, 10 or 30 μg/kg (dose levels 1–3; DL1–3) by intravenous infusion on a Q2W schedule. Step-up dosing was implemented starting at DL4 where patients received 2 or 3 priming doses prior to the target dose of 60 μg/kg (DL4), 90 μg/kg (DL5) or 120 μg/kg (DL6). Primary endpoints include incidence and severity of adverse events (AEs). Secondary endpoints include assessment of PK, PD and tumor response per RECISTv1.1 and iRECIS.
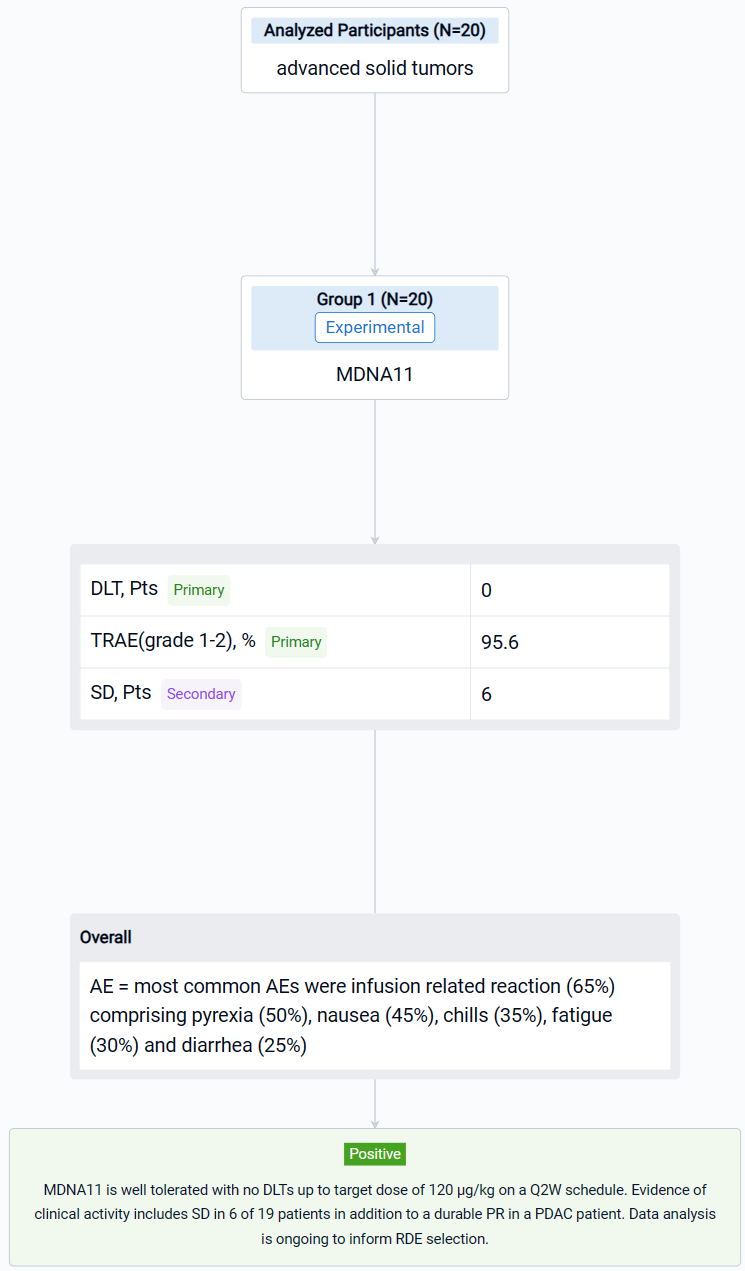
The result showed that as of June 22, 2023, twenty dose limiting toxicity (DLT)-evaluable patients have been dosed with MDNA11 during monotherapy dose escalation (3–120 μg/kg). Tumor types enrolled included melanoma (n=11), renal cell carcinoma (n=2), pancreatic ductal adenocarcinoma (PDAC; n=2), sarcoma (n=2), tonsillar squamous cell carcinoma (n=1), gastro-esophageal adenocarcinoma (n=1) and lung adenocarcinoma (n=1). PK analysis showed dose-dependent increase in MDNA11 exposure. Immune profiling showed robust lymphocyte expansion, including increase in CD8+ T and NK cells and limited change in Tregs. There were no DLTs observed. The most common AEs were infusion related reaction (65%) comprising pyrexia (50%), nausea (45%), chills (35%), fatigue (30%) and diarrhea (25%), with the majority being grade 1–2 and resolved within 48–72 hours. Transient (~ 1week duration) transaminases increase was seen in 25% of patients. Tumor response was evaluated in 19 patients. Single-agent activity included stable disease (SD) observed in 6 patients, including a melanoma patient with SD beyond 1.5 year, and an ongoing partial response (PR) in a PDAC patient who had previously progressed on immune checkpoint inhibitor and is currently continuing on MDNA11 for > 1 year.
It can be concluded that MDNA11 is well tolerated with no DLTs up to target dose of 120 μg/kg on a Q2W schedule. Evidence of clinical activity includes SD in 6 of 19 patients in addition to a durable PR in a PDAC patient. Data analysis is ongoing to inform RDE selection.
How to Easily View the Clinical Results Using Synapse Database?
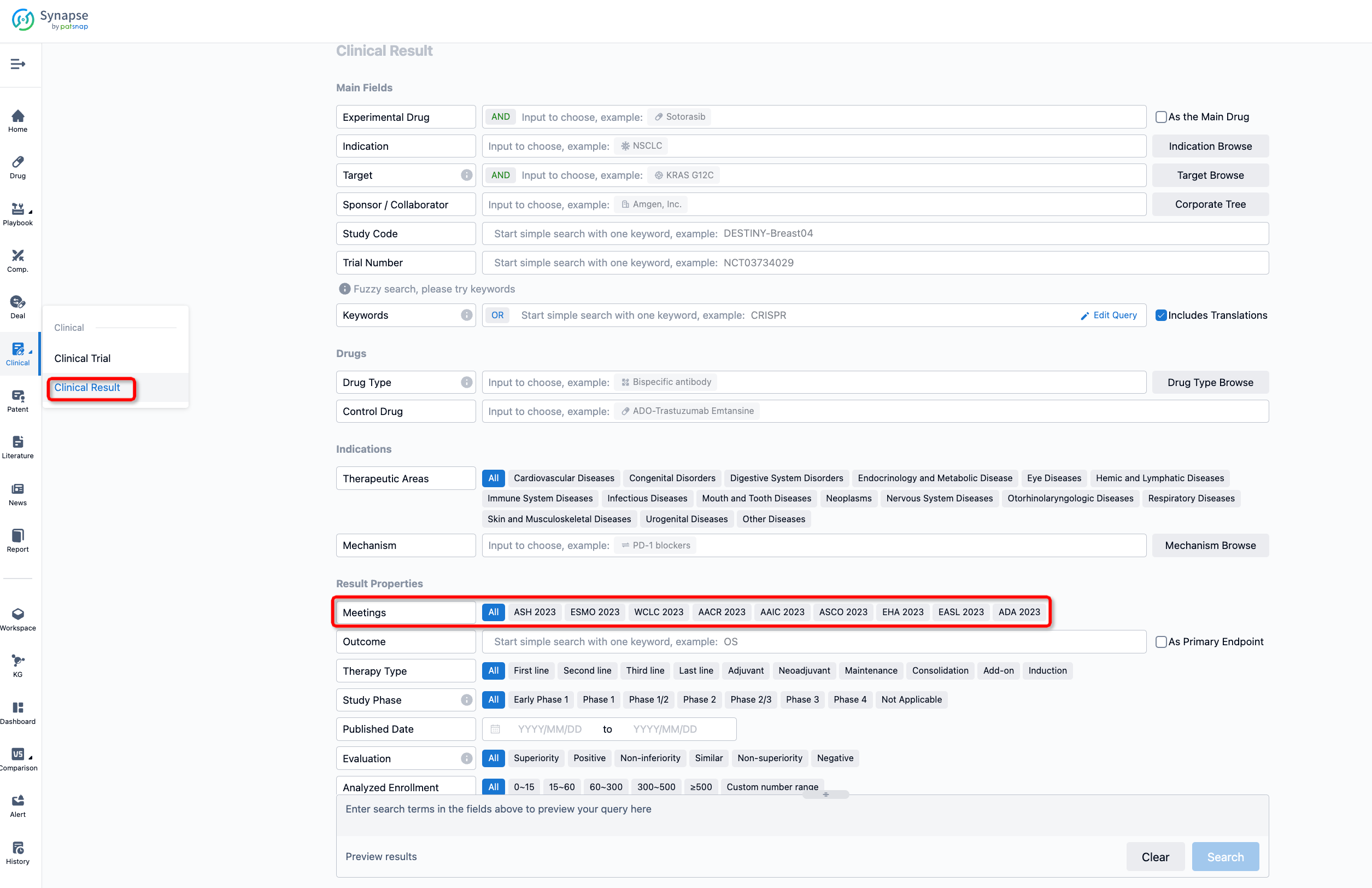
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
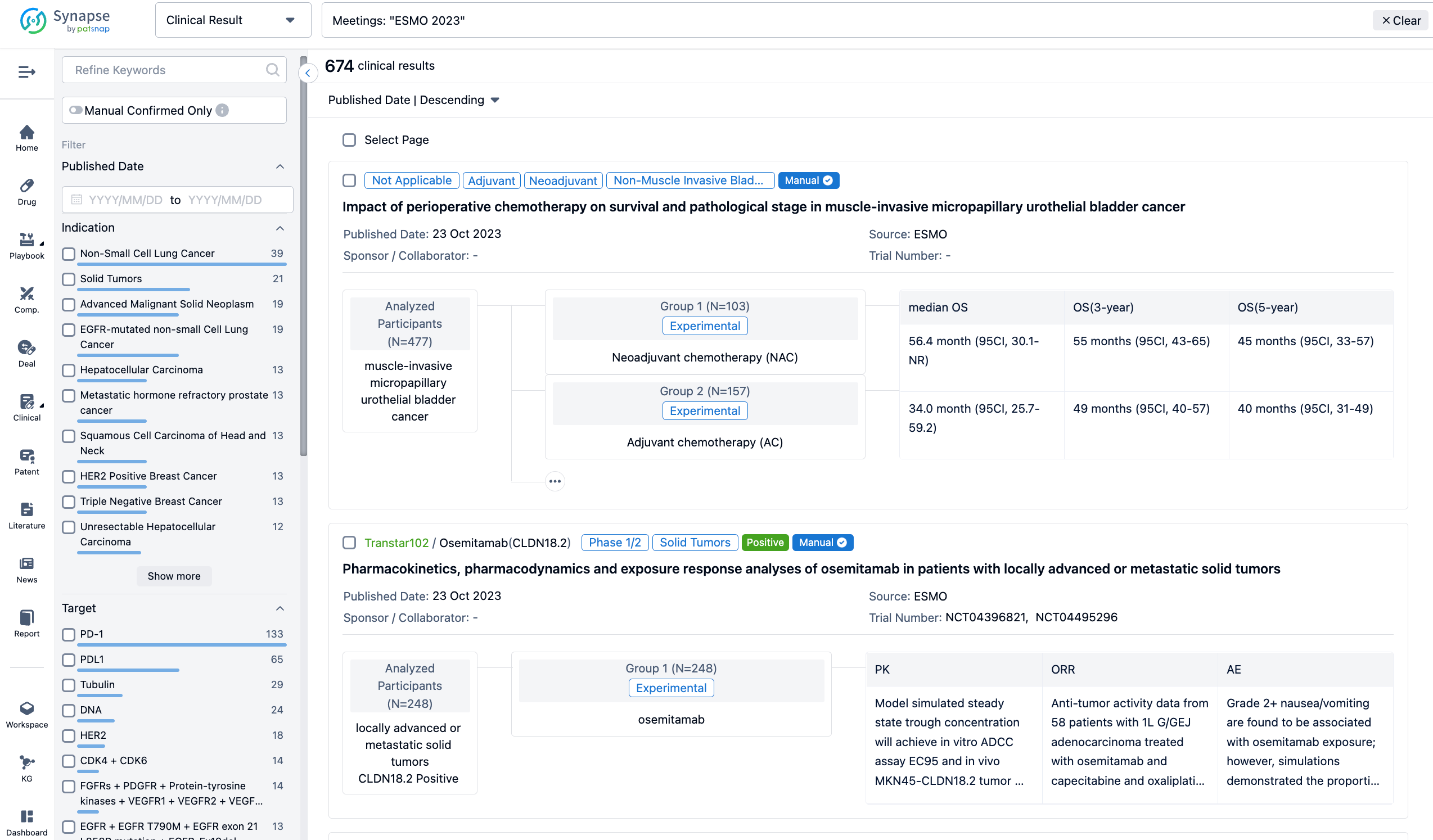
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
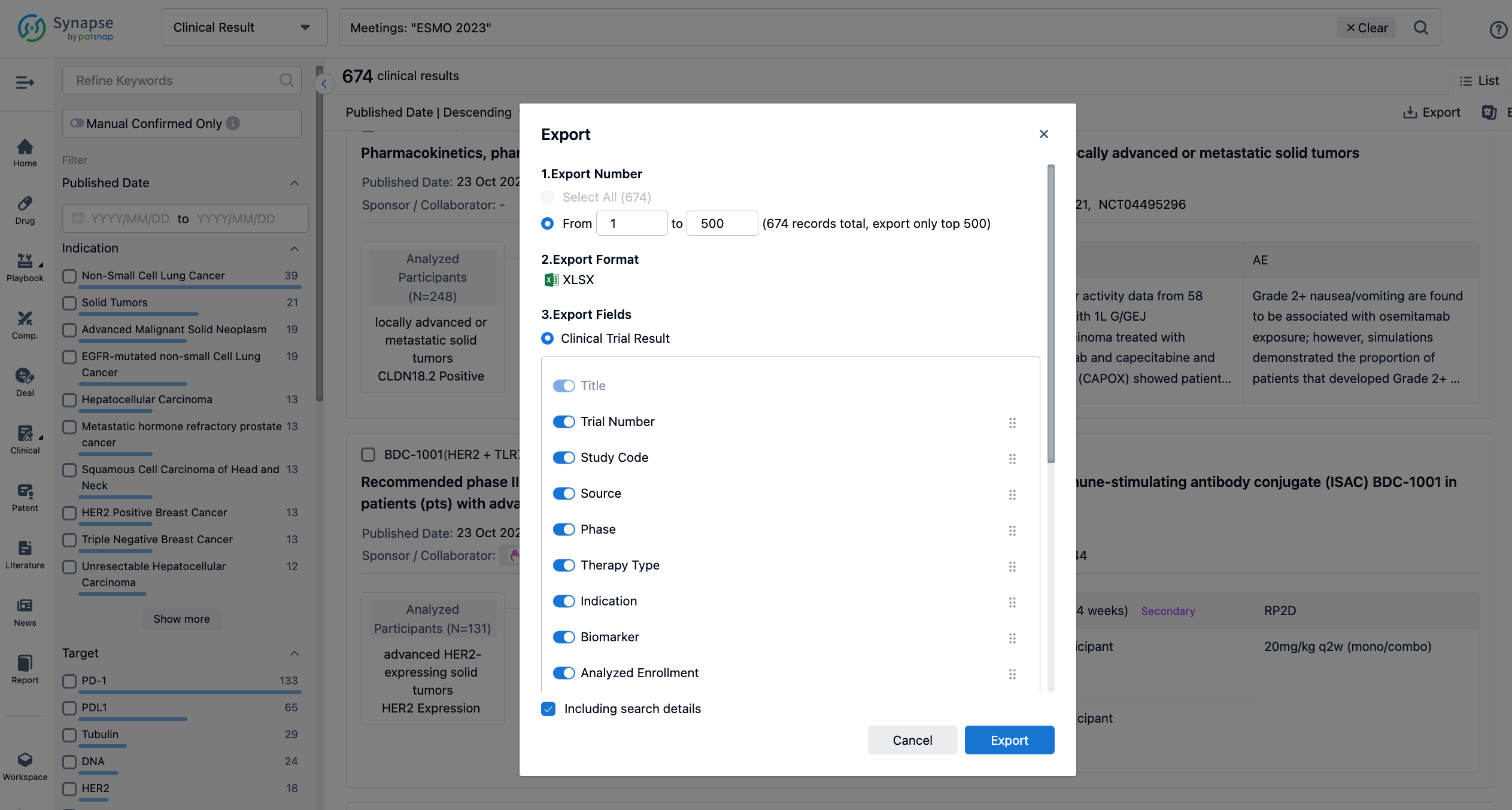
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!