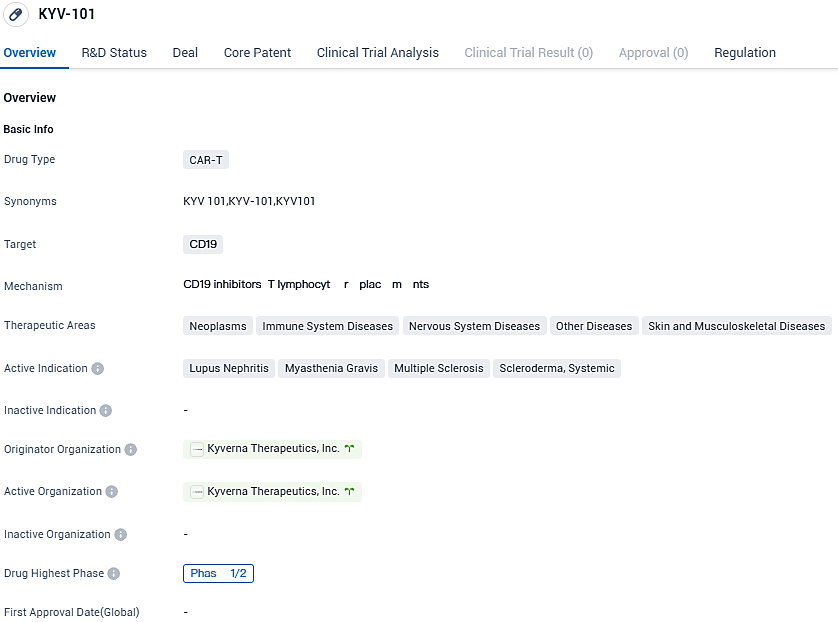
Kyverna Therapeutics announces Phase 2 trial approval for KYV-101, a full human CD19 CAR T-Cell therapy for Myasthenia Gravis, by the FDA
Kyverna Therapeutics, Inc., a clinical-stage biopharmaceutical company with a patient-oriented approach to developing cell therapies for those afflicted with autoimmune disorders, has stated that its fifth IND submission for KYV-101 has obtained approval from FDA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Kyverna is now authorized to commence a Phase 2 open-label, multicenter trial of KYV-101, a human CD19 chimeric antigen receptor T cell product candidate fully autologous, intended to treat myasthenia gravis patients. The clinical study, termed KYSA-6, broadens Kyverna's ongoing initiatives, such as the U.S. based Phase 1 KYSA-1 study and the Phase 1/2 KYSA-3 study happening in Germany, wherein the efficacy of KYV-101 is being examined in adults afflicted with active lupus nephritis.
Also ongoing is the Phase 1/2 KYSA-5 study for adults with diffuse cutaneous systemic sclerosis within the U.S. Kyverna has additionally secured clearance for an extra pair of INDs for KYV-101 investigator-driven trials within the U.S. Prof. Aiden Haghikia, Head of Neurology Department at Otto-von-Guericke University, Magdeburg, Germany stated, "We have witnessed firsthand how KYV-101 can transform the lives of MG patients in our clinic.
I welcome this decision by the FDA and anticipate more clinical data to broaden our understanding of CAR T-cell therapy within patients with severe neurological autoimmune disorders." Peter Maag, Ph.D., CEO of Kyverna, expressed, "We are appreciative of the FDA's approval of our IND for Phase 2 KYSA-6 trial. This will facilitate Kyverna to provide this investigational treatment, which could alter the paradigm, to patients likely to see benefits from deep B cell depletion and possible durable immune system reset."
Kyverna's CAR T-cell therapy involves adapting a patient's T cells to identify and eliminate B cells in the body. KD19 CAR T-cell therapy, KYV-101, particularly targets CD19, a protein shown on the surface of B cells, playing a part in multiple autoimmune diseases. Kyverna intends to expand the applications for KYV-101 and develop a strong pipeline of potential immunotherapies that address the unmet medical demands in autoimmune diseases.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
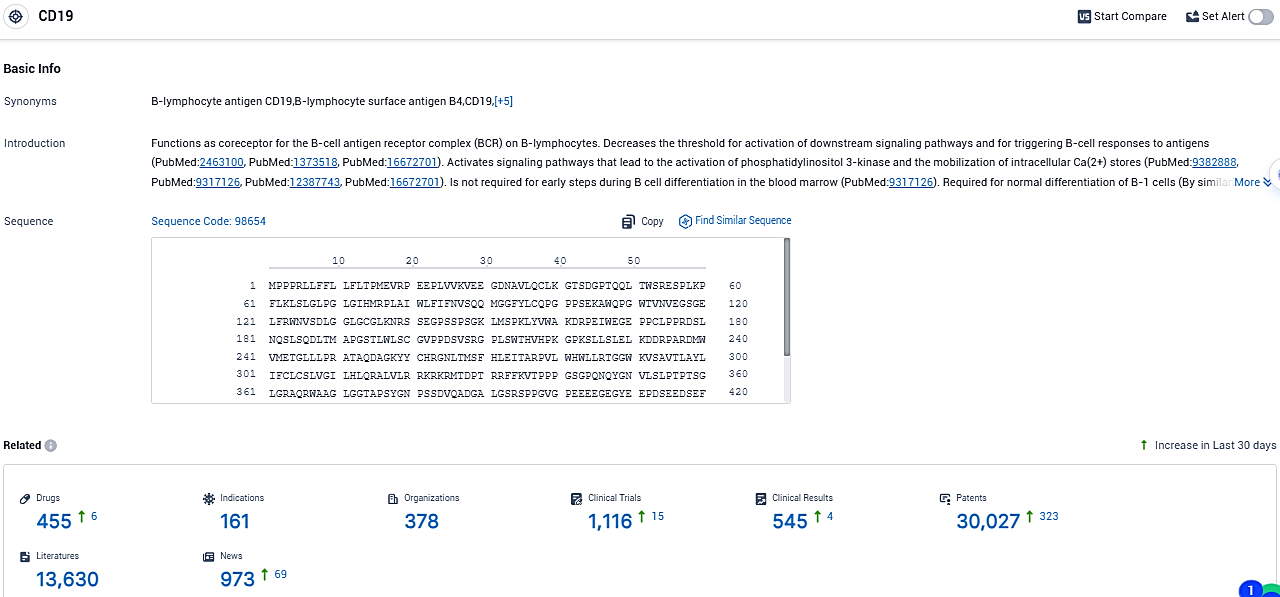
According to the data provided by the Synapse Database, As of November 21, 2023, there are 455 investigational drugs for the CD19 target, including 161 indications, 378 R&D institutions involved, with related clinical trials reaching 1116, and as many as 30027 patents.
KYV-101 is an autologous, fully human CD19 CAR T-cell product candidate for use in B cell-driven autoimmune diseases. The CAR in KYV-101 was designed by NIH to improve tolerability and tested in a 20-patient Phase 1 trial in oncology. Results were published by the NIH in Nature Medicine.