An In-depth Analysis of Icosapent Ethyl's R&D Progress
Icosapent Ethyl's R&D Progress
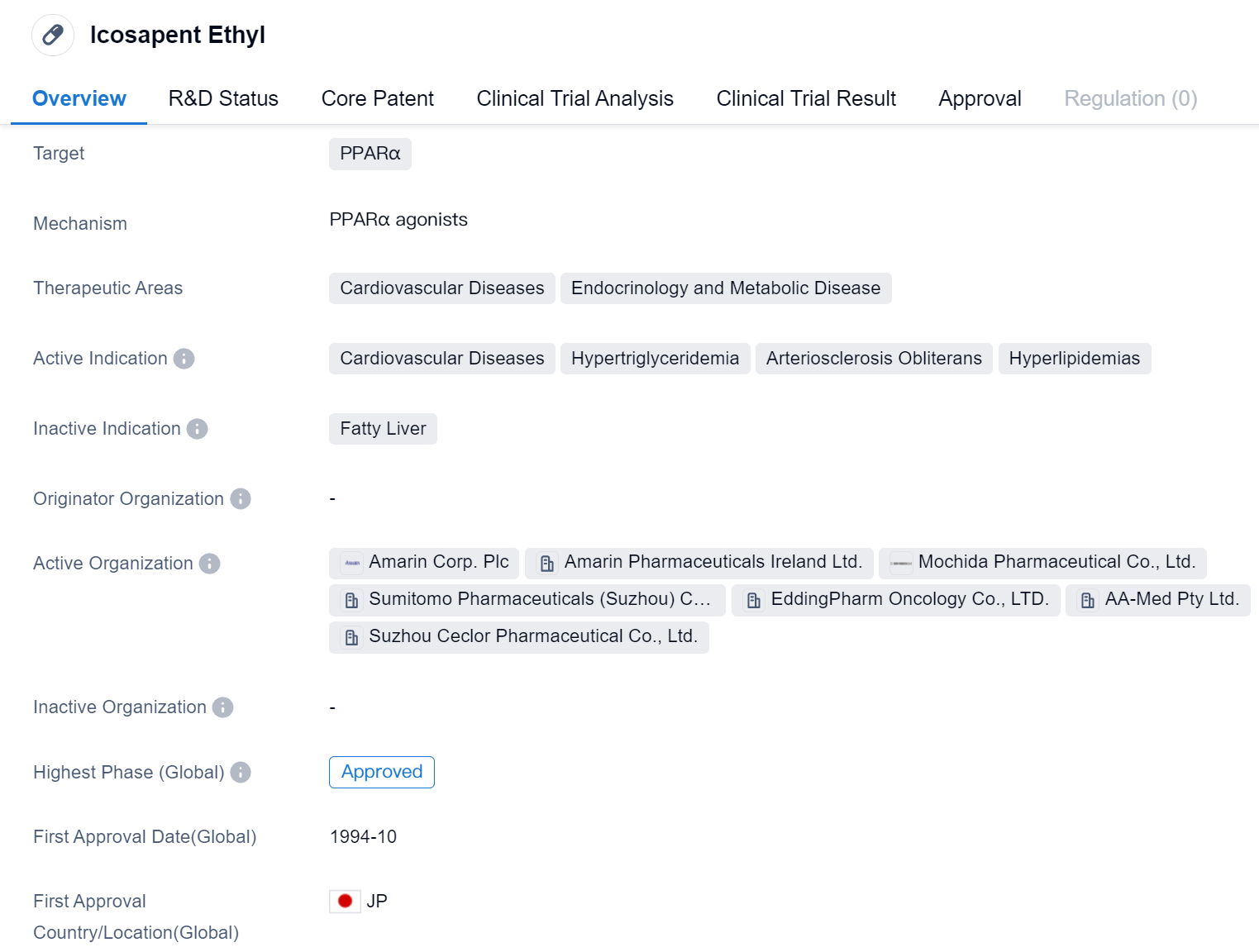
Icosapent Ethyl is a small molecule drug that falls under the category of biomedicine. It specifically targets PPARα, which stands for Peroxisome proliferator-activated receptor alpha. This drug is primarily used in the treatment of cardiovascular diseases, endocrinology, and metabolic diseases.
The active indications for Icosapent Ethyl include cardiovascular diseases, hypertriglyceridemia, arteriosclerosis obliterans, and hyperlipidemias. These conditions are often associated with high levels of triglycerides and cholesterol in the blood, which can lead to the development of cardiovascular problems such as heart disease and stroke.
The drug was first approved in Japan in October 1994, making it available for use in that country. In terms of its development, Icosapent Ethyl has reached the highest phase of development which is approved globally.
As a small molecule drug, Icosapent Ethyl is designed to interact with specific molecular targets in the body, in this case, PPARα. By targeting this receptor, the drug aims to modulate the activity of genes involved in lipid metabolism and inflammation, ultimately leading to a reduction in triglyceride levels and improved cardiovascular health.
The approval of Icosapent Ethyl in multiple countries and its indication for various cardiovascular conditions highlight its potential as an effective treatment option. Its approval in China, a significant market in the pharmaceutical industry, further emphasizes its potential for widespread use and commercial success.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Icosapent Ethyl: PPARα agonists
From a biomedical perspective, PPARα agonists are a type of drug that activate the peroxisome proliferator-activated receptor alpha (PPARα) in the body. PPARα is a nuclear receptor that plays a crucial role in regulating lipid metabolism and inflammation. By activating PPARα, these agonists can increase the expression of genes involved in fatty acid oxidation and decrease the expression of genes involved in lipid synthesis. This can lead to improved lipid profiles and reduced inflammation, making PPARα agonists useful in the treatment of conditions such as dyslipidemia, hypertriglyceridemia, and metabolic syndrome. Some examples of PPARα agonists include fenofibrate and gemfibrozil.
Drug Target R&D Trends for Icosapent Ethyl
PPARα, or Peroxisome Proliferator-Activated Receptor Alpha, is a nuclear receptor that plays a crucial role in regulating lipid metabolism in the human body. It is primarily expressed in tissues involved in fatty acid catabolism, such as the liver, heart, and skeletal muscles. PPARα activation leads to increased fatty acid oxidation, resulting in the breakdown of triglycerides and the generation of energy. This receptor also influences glucose homeostasis, inflammation, and lipid transport. Due to its involvement in lipid metabolism, PPARα has become a target for pharmaceutical interventions aimed at treating dyslipidemia, metabolic disorders, and cardiovascular diseases.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 127 PPARα drugs worldwide, from 153 organizations, covering 110 indications, and conducting 906 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Icosapent Ethyl is a small molecule drug that targets PPARα and is primarily used in the treatment of cardiovascular diseases, endocrinology, and metabolic diseases. It has been approved in multiple countries, including Japan and China, and has shown promise in reducing triglyceride levels and improving cardiovascular health. Its long history of approval and its indication for various cardiovascular conditions make it a valuable asset in the pharmaceutical industry.