An In-depth Analysis of imiglucerase's R&D Progress and Mechanism of Action on Drug Target
Imiglucerase's R&D Progress
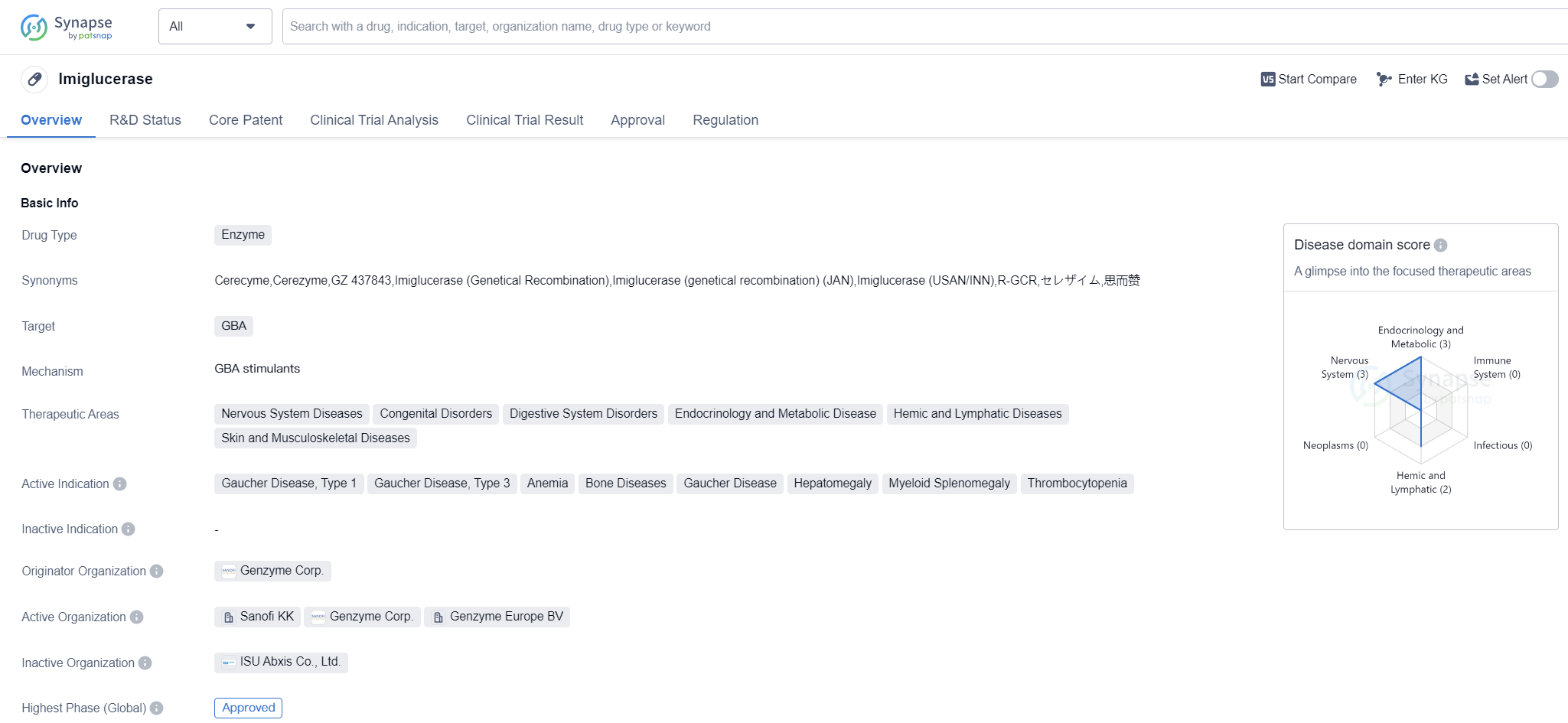
Imiglucerase is an enzyme-based drug that targets the GBA enzyme. It is primarily used in the treatment of various diseases related to the nervous system, congenital disorders, digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The active indications for Imiglucerase include Gaucher Disease, Type 1 and Type 3, anemia, bone diseases, Gaucher Disease, hepatomegaly, myeloid splenomegaly, and thrombocytopenia.
The drug was developed by Genzyme Corp., a renowned pharmaceutical company. Imiglucerase has received approval for use in multiple countries, including the United States, where it was first approved in May 1994. It is classified as an orphan drug, indicating that it is used to treat rare diseases or conditions.
Imiglucerase's mechanism of action involves replacing the deficient or malfunctioning GBA enzyme in patients with Gaucher Disease. Gaucher Disease is a rare genetic disorder characterized by the accumulation of a fatty substance called glucocerebroside in certain organs and tissues. This accumulation can lead to various symptoms, including anemia, bone pain, enlarged liver and spleen, and low platelet count.
The approval of Imiglucerase in multiple countries highlights its effectiveness and safety profile. It has undergone rigorous clinical trials and regulatory processes to ensure its quality and efficacy. Being an orphan drug, Imiglucerase addresses an unmet medical need for patients suffering from Gaucher Disease and related conditions.
The approval of Imiglucerase in China further expands its availability and accessibility to patients in the country. This signifies the drug's potential to benefit a larger population and improve the management of Gaucher Disease in China.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for imiglucerase: GBA stimulants
GBA stimulants are a type of medication that act as stimulants for the GBA (glucocerebrosidase) enzyme. Glucocerebrosidase is an enzyme involved in the breakdown of a fatty substance called glucocerebroside. When there is a deficiency or dysfunction of this enzyme, it can lead to the accumulation of glucocerebroside in cells, which is associated with certain genetic disorders such as Gaucher disease.
GBA stimulants work by enhancing the activity of the GBA enzyme, promoting the breakdown of glucocerebroside and reducing its accumulation in cells. By increasing the enzyme's activity, these stimulants aim to alleviate the symptoms and slow down the progression of Gaucher disease.
It's important to note that GBA stimulants are a specific type of medication used in the context of Gaucher disease or other conditions related to GBA enzyme dysfunction. They are not a general term for all types of stimulants or medications.
Drug Target R&D Trends for imiglucerase
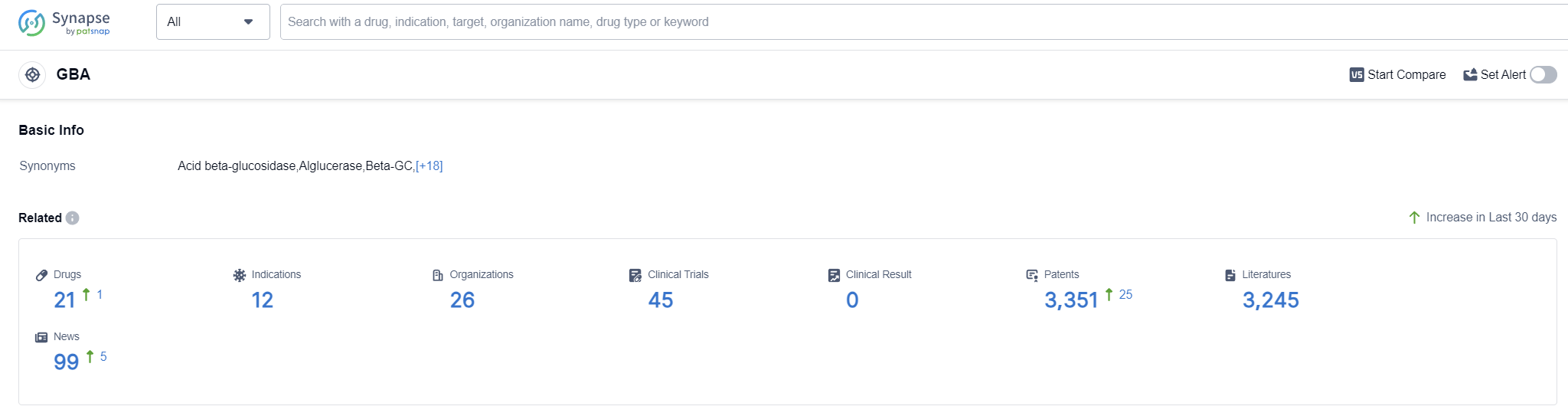
The GBA (glucocerebrosidase) enzyme plays a crucial role in the human body by breaking down a fatty substance called glucocerebroside. This substance is found in various cells and tissues, particularly in the spleen, liver, and bone marrow. GBA deficiency leads to the accumulation of glucocerebroside, causing a group of rare genetic disorders known as Gaucher disease. Gaucher disease can manifest in different ways, affecting the liver, spleen, bones, and other organs. Understanding the role of GBA and its deficiency is essential for developing therapeutic interventions and treatments for Gaucher disease and potentially other related conditions.
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 21 GBA drugs worldwide, from 26 organizations, covering 12 indications, and conducting 45 clinical trials.
The analysis of the target GBA reveals a competitive landscape with multiple companies actively involved in the development of drugs. ISU Abxis Co., Ltd., Pfizer Inc., and Sanofi are among the companies with drugs in the approved stage. Gaucher Disease is the most common indication, with drugs approved and in various stages of development. Enzyme, biosimilars, and small molecule drugs are progressing rapidly, indicating intense competition. The United States is leading in terms of drug development, followed by the European Union and China. China has shown progress with one drug approved and two drugs in the preclinical stage. Overall, the target GBA presents opportunities for innovation and competition in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Imiglucerase is an enzyme-based drug developed by Genzyme Corp. It is used to treat various diseases related to the nervous system, congenital disorders, digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. Its primary indication is Gaucher Disease, and it has been approved in multiple countries, including the United States and China. Imiglucerase's approval as an orphan drug highlights its importance in addressing rare diseases and improving patient outcomes.