An In-depth Analysis of Letermovir's R&D Progress and Mechanism of Action on Drug Target
Letermovir's R&D Progress
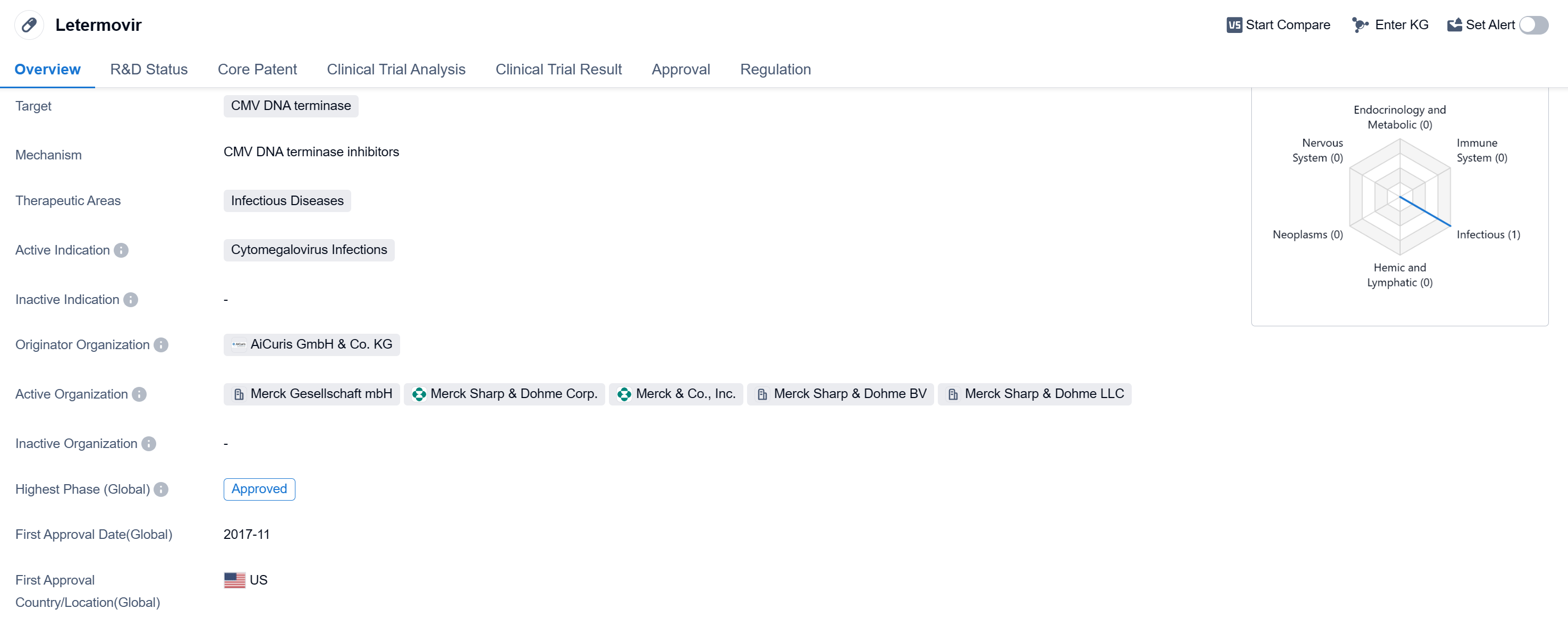
Letermovir is a small molecule drug that falls under the therapeutic area of infectious diseases. It specifically targets CMV DNA terminase, making it effective against cytomegalovirus (CMV) infections. The drug has been approved in both the global market and China.
The originator organization of Letermovir is AiCuris GmbH & Co. KG. It received its first approval in the United States in November 2017, marking its entry into the global market. The drug has undergone rigorous regulatory processes and has been granted several designations, including Priority Review, Fast Track, Breakthrough Therapy, and Orphan Drug status.
CMV is a common virus that can cause severe complications, especially in individuals with weakened immune systems, such as transplant recipients and those with HIV/AIDS. Letermovir's ability to target CMV DNA terminase makes it a promising option for patients suffering from CMV infections.
The small molecule nature of Letermovir suggests that it is a chemically synthesized compound rather than a biological product. This characteristic may offer advantages in terms of manufacturing, stability, and ease of administration.
The approval of Letermovir in the globa markets highlights its potential to address the unmet medical needs of patients with CMV infections. The drug's designations as Priority Review, Fast Track, Breakthrough Therapy, and Orphan Drug further emphasize its importance in the field of infectious diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Letermovir: CMV DNA terminase inhibitors
CMV DNA terminase inhibitors are a type of antiviral drugs that specifically target the DNA terminase enzyme of cytomegalovirus (CMV). Cytomegalovirus is a common virus that can cause severe complications in individuals with weakened immune systems, such as transplant recipients or people with HIV/AIDS.
DNA terminase is an essential enzyme for the replication of CMV DNA. It plays a crucial role in cutting and packaging the viral DNA into new viral particles during the viral replication process. CMV DNA terminase inhibitors work by blocking the activity of this enzyme, preventing the proper assembly and release of new infectious CMV particles.
By inhibiting CMV DNA terminase, these drugs can effectively inhibit the replication of cytomegalovirus, reducing the viral load and potentially preventing the progression of CMV-related diseases. They are primarily used in the treatment and prevention of CMV infections in immunocompromised patients.
It's important to note that CMV DNA terminase inhibitors are a specific type of antiviral drug and should not be confused with other classes of antivirals or general DNA terminase inhibitors used in different contexts.
Drug Target R&D Trends for Letermovir
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 3 CMV DNA terminase drugs worldwide, from 9 organizations, covering 6 indications, and conducting 42 clinical trials.
The analysis of the current competitive landscape of the target CMV DNA terminase reveals that Merck & Co., Inc. and Merck KGaA are leading in terms of development phase and have multiple drugs in different stages of development. The approved drugs under this target are indicated for Cytomegalovirus Infections. Small molecule drugs are progressing rapidly, with one approved and one preclinical drug. The development of drugs under this target is spread across various countries, including the United States, China, and South Korea. The future development of the target CMV DNA terminase shows potential for advancements in the treatment of Cytomegalovirus Infections and other indications such as Tuberculosis and COVID-19.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Letermovir is a small molecule drug developed by AiCuris GmbH & Co. KG. It targets CMV DNA terminase and has been approved for the treatment of CMV infections in the United States and China. The drug's regulatory designations and its therapeutic potential make it a significant advancement in the field of infectious diseases.