An In-depth Analysis of proxalutamide's R&D Progress and Mechanism of Action on Drug Target
proxalutamide's R&D Progress
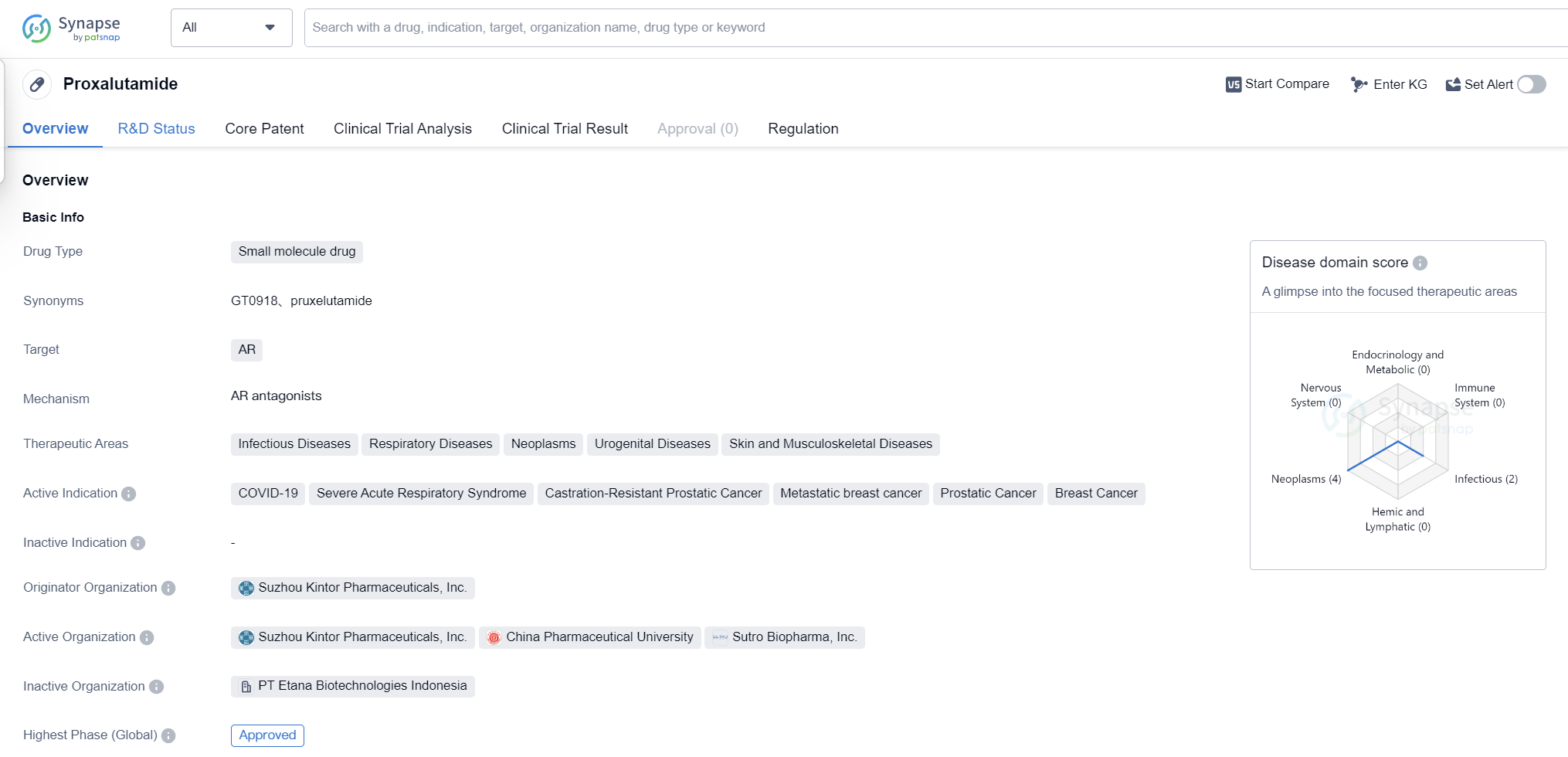
Proxalutamide is a small molecule drug that targets the androgen receptor (AR). It has shown potential in treating various therapeutic areas, including infectious diseases, respiratory diseases, neoplasms, urogenital diseases, and skin and musculoskeletal diseases. The drug has been indicated for the treatment of several conditions, including COVID-19, severe acute respiratory syndrome, castration-resistant prostatic cancer, metastatic breast cancer, prostatic cancer, and breast cancer.
The originator organization of Proxalutamide is Suzhou Kintor Pharmaceuticals, Inc. The drug has reached the highest phase of development which is approved globally. In China, it is currently in Phase 3 of clinical trials. Proxalutamide received its first approval in Paraguay in July 2021, making it available for use in that country.
In terms of regulation, Proxalutamide has been granted Emergency Use Authorization and is also part of a Special Review Project. These regulatory measures indicate that the drug has been fast-tracked for approval and use, likely due to the urgent need for effective treatments in the indicated therapeutic areas.
Proxalutamide's approval for COVID-19 treatment is particularly noteworthy, as the ongoing pandemic has created a high demand for effective drugs to combat the disease. The drug's ability to target the androgen receptor may play a crucial role in its efficacy against COVID-19, as androgens have been implicated in the severity of the disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for proxalutamide: AR antagonists
AR antagonists, also known as androgen receptorantagonists, are a class of drugs that inhibit the activity ofthe androgen receptor. The androgen receptor is a proteinfound in cells that binds to androgens, such astestosterone and dihydrotestosterone. By blocking theandrogen receptor, AR antagonists prevent androgensfrom exerting their effects on the body.
In the context of biomedicine, AR antagonists are primarilyused in the treatment of conditions that are influenced byandrogens, such as prostate cancer. Prostate cancer cellsoften rely on androgens for growth and survival. Byinhibiting the androgen receptor, AR antagonists can slowdown or halt the growth of prostate cancer cells.
AR antagonists are also used in the management of otherandrogen-related conditions, such as androgeneticalopecia (male pattern baldness) and hirsutism (excessivehair growth). In these cases, the goal is to reduce theeffects of androgens on the target tissues.
It's important to note that AR antagonists can have sideeffects, as androgens play a role in various physiologicalprocesses.Common side effects may include fatigue, hotflashes, decreased libido, and changes in bone density.The specific AR antagonist used and the individualpatient's response to the medication will determine theextent and severity of side effects.
Drug Target R&D Trends for proxalutamide
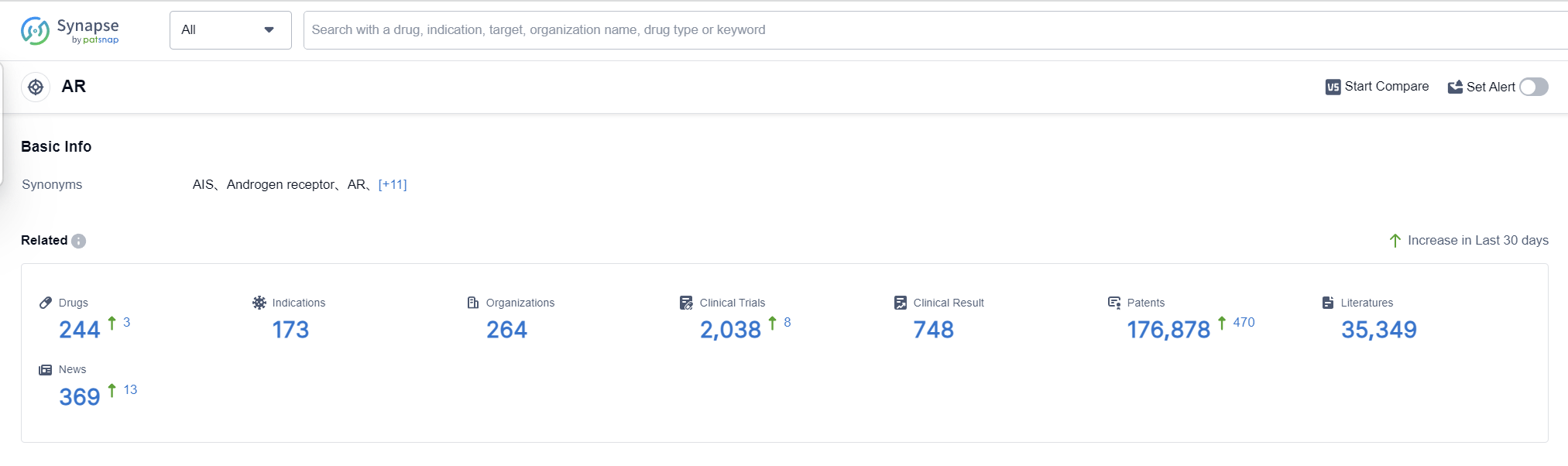
AR, or Androgen Receptor, plays a crucial role in the human body. It is a protein found in cells that binds to androgens, such as testosterone and dihydrotestosterone. Once bound, AR regulates gene expression, leading to the development and maintenance of male sexual characteristics. Additionally, AR is involved in various physiological processes, including bone and muscle growth, hair growth, and the regulation of mood and cognition. Dysregulation of AR activity can contribute to several diseases, including prostate cancer and androgen insensitivity syndrome. Understanding the role of AR is essential for developing targeted therapies and improving patient outcomes in the pharmaceutical industry.
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 244 AR drugs worldwide, from 264 organizations, covering 173 indications, and conducting 2038 clinical trials.
The current competitive landscape in the target AR is characterized by the presence of several key companies, including Bayer AG, Pfizer Inc., Suzhou Kintor Pharmaceuticals, Inc., and Johnson & Johnson. These companies have a strong focus on research and development, with a diverse pipeline of drugs in various stages of development.
The most common indications for approved drugs in the target AR are Prostatic Cancer, Hypogonadism, Breast Cancer, Acne Vulgaris, and Contraception. This indicates a significant research focus on developing treatments for these conditions.
Small molecule drugs and PROTACs are the drug types that are progressing most rapidly under the current target AR. The development of small molecule drugs is highly competitive, indicating intense competition around innovative drugs. Other drug types, such as antisense oligonucleotides, therapeutic vaccines, and DNA vaccines, are also being explored.
The United States, China, and the European Union are the countries/locations that are developing fastest under the current target AR. China, in particular, has shown significant progress in the development of drugs for the target AR, indicating a growing research focus in the region.
Overall, the target AR presents a competitive landscape with active research and development efforts from key companies and a focus on developing treatments for specific indications. The future development of the target AR is likely to be driven by advancements in small molecule drugs, PROTACs, and ongoing research in countries like China.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Proxalutamide shows promise as a versatile drug with potential applications in various therapeutic areas. Its approval for COVID-19 treatment and its progress in clinical trials for other conditions like prostatic and breast cancer highlight its potential impact on public health. As more data becomes available from ongoing trials and real-world use, further insights into the drug's effectiveness and safety profile will be gained.