An In-depth Analysis of serplulimab's R&D Progress and Mechanism of Action on Drug Target
Serplulimab's R&D Progress
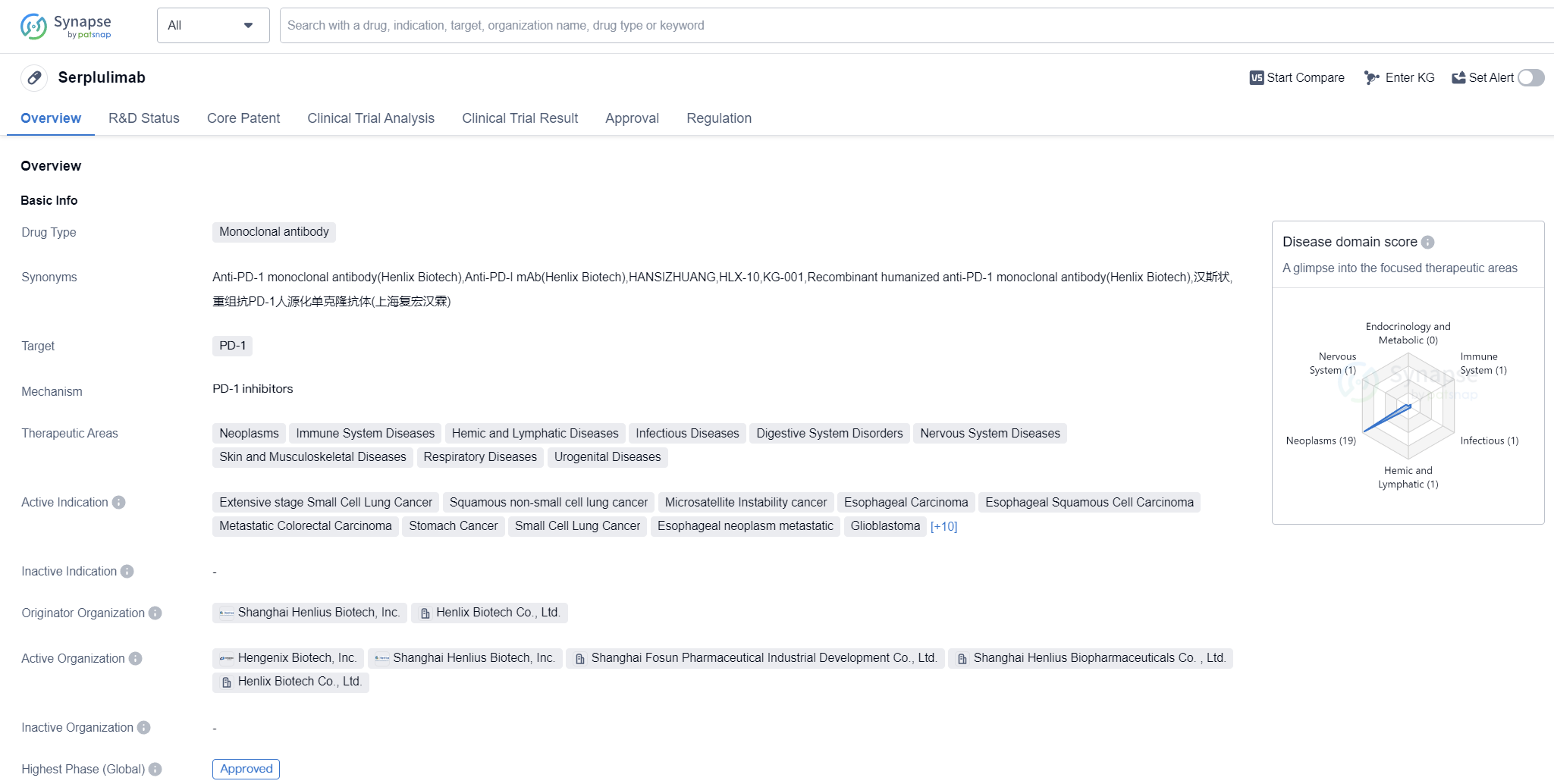
Serplulimab is a monoclonal antibody drug that targets PD-1, a protein involved in regulating the immune system. It has been developed by Shanghai Henlius Biotech, Inc. and Henlix Biotech Co., Ltd. This drug falls under the therapeutic areas of neoplasms, immune system diseases, hemic and lymphatic diseases, infectious diseases, digestive system disorders, nervous system diseases, skin and musculoskeletal diseases, respiratory diseases, and urogenital diseases.
Serplulimab has shown potential in treating various types of cancers and other diseases. It has been indicated for extensive stage small cell lung cancer, squamous non-small cell lung cancer, microsatellite instability cancer, esophageal carcinoma, esophageal squamous cell carcinoma, metastatic colorectal carcinoma, stomach cancer, small cell lung cancer, esophageal neoplasm metastatic, glioblastoma, uterine cervical cancer, colorectal cancer, hepatocellular carcinoma, triple-negative breast cancer, non-squamous non-small cell lung cancer, squamous cell carcinoma of the head and neck, hepatitis B (chronic), liver cancer, lymphoma, and solid tumors.
The drug has reached the highest phase of development which is approved globally. The first approval for Serplulimab was granted in China in March 2022. It is worth noting that the drug has undergone priority review and has been designated as an orphan drug, indicating its potential to address unmet medical needs for rare diseases.
Serplulimab's approval and its broad therapeutic areas suggest its potential to make a significant impact in the field of biomedicine. By targeting PD-1, it aims to enhance the body's immune response against cancer cells and other diseases. The highest R&D phase of this drug is approved.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for serplulimab: PD-1 inhibitors
PD-1 inhibitors are a type of medication used in biomedicine to treat certain types of cancer. PD-1 stands for programmed cell death protein 1, which is a protein found on the surface of immune cells called T cells. PD-1 plays a role in regulating the immune system and preventing it from attacking healthy cells in the body.
In cancer, tumor cells can exploit the PD-1 pathway to evade the immune system's response. They can express a protein called PD-L1, which binds to PD-1 on T cells and sends inhibitory signals, suppressing the immune response against the tumor. This allows the tumor to grow and evade destruction by the immune system.
PD-1 inhibitors work by blocking the interaction between PD-1 and PD-L1, thereby releasing the brakes on the immune system. By doing so, these inhibitors enhance the ability of the immune system to recognize and attack cancer cells. They help to restore the immune response against the tumor, leading to tumor regression and improved patient outcomes.
PD-1 inhibitors have shown significant efficacy in the treatment of various cancers, including melanoma, non-small cell lung cancer, renal cell carcinoma, and Hodgkin's lymphoma. They are often used as a form of immunotherapy and can be administered alone or in combination with other anticancer agents.
It is important to note that PD-1 inhibitors can have side effects, as they can also unleash the immune system against healthy tissues, leading to immune-related adverse events. Common side effects include fatigue, rash, diarrhea, and inflammation of organs such as the lungs, liver, or intestines. Close monitoring and management of these side effects are necessary during treatment.
Overall, PD-1 inhibitors have revolutionized the field of cancer treatment by harnessing the power of the immune system to fight against cancer cells. They have provided new hope for patients with advanced or metastatic cancers and have significantly improved survival rates in certain malignancies.
Drug Target R&D Trends for serplulimab
PD-1, or programmed cell death protein 1, is a crucial immune checkpoint receptor found on the surface of certain immune cells in the human body. Its primary role is to regulate the immune response and prevent excessive activation of immune cells, thereby maintaining immune homeostasis. PD-1 acts as a brake on the immune system by binding to its ligands, PD-L1 and PD-L2, which are expressed on various cells, including cancer cells. This interaction inhibits the immune response, allowing cancer cells to evade detection and destruction by the immune system. Targeting the PD-1 pathway has revolutionized cancer treatment, leading to the development of immune checkpoint inhibitors that unleash the immune system's ability to fight cancer.
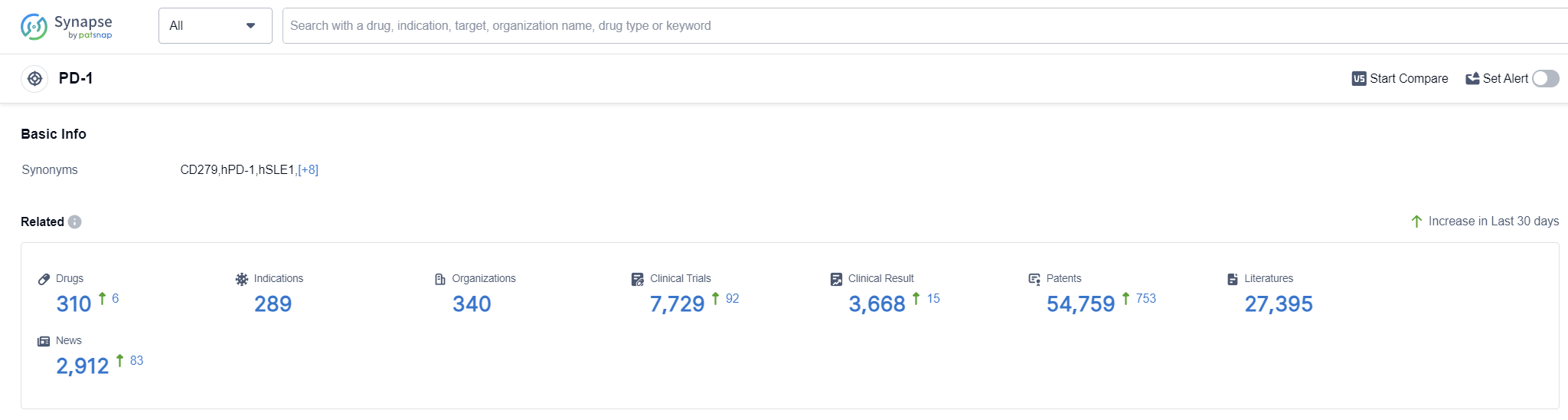
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 310 PD-1 drugs worldwide, from 340 organizations, covering 289 indications, and conducting 7729 clinical trials.
The analysis of the target PD-1 reveals a competitive landscape with several companies actively developing PD-1 inhibitors. Akeso, Inc., Bristol Myers Squibb Co., and Merck & Co., Inc. are the leading companies in terms of R&D progress. The most common indications for PD-1 inhibitors include Hodgkin's Lymphoma, Non-Small Cell Lung Cancer, Melanoma, and Esophageal Squamous Cell Carcinoma. Monoclonal antibodies are the dominant drug type, followed by bispecific antibodies and biosimilars. China, the United States, and the European Union are the key locations for PD-1 inhibitor development, with China showing significant progress. Overall, the target PD-1 presents a competitive market with promising future development opportunities.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Serplulimab's approval and its wide range of therapeutic indications make it a promising addition to the pharmaceutical industry's arsenal against various diseases. Its success in clinical trials and regulatory approvals demonstrate its potential to improve patient outcomes and address unmet medical needs.