Oncternal Therapeutics begins phase 1/2 trial of ONCT-534, a dual-action AR inhibitor, on metastatic castration-resistant prostate cancer patients
Oncternal Therapeutics, Inc., an emerging biopharmaceutical firm in the clinical phase concentrating on innovative cancer therapies, has reported the commencement of its Phase 1/2 dose escalation/dose expansion trial with the first patient receiving the ONCT-534, their pioneering bi-function androgen receptor suppressor.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Salim Yazji, Oncternal's Chief Medical Officer, expressed that administering ONCT-534 to the first patient is a crucial step forward for those battling advanced prostate cancer who have not responded or have regressed post AR signaling inhibitors treatment. He further added, studies prior to the clinical trials point towards ONCT-534 potentially overturning major tumor evasion mechanisms causing resistance against available AR inhibitors like enzalutamide or abiraterone. Patient recruitment from various U.S. locations will be carried out in the ensuing weeks, and from the United Kingdom by early 2024. Preliminary clinical data is expected to be available in the first half of 2024.
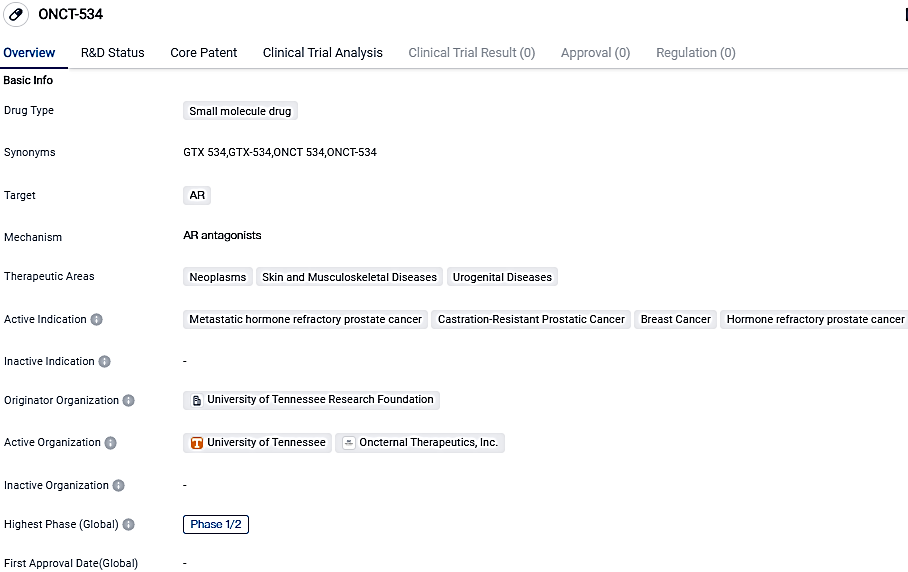
ONCT-534, a dual-action androgen receptor inhibitor, interferes with both the N-terminal domain and the ligand-binding domain of the androgen receptor, thereby inhibiting cell proliferation and triggering AR degradation. Preclinical data has demonstrated its potential against both unaltered AR and multiple mutations, inclusive of AR amplification, mutations in the AR LBD, and splice variants sans AR LBD in prostate cancer models.
The research ONCT-534-101 is a Phase 1/2, single-arm, open-label, multi-center analysis which aims to assess the safety, tolerability, pharmacokinetics, and anti-tumor effects of ONCT-534 in patients with metastatic castration-resistant prostate cancer who have not derived benefit or are refractory to approved androgen receptor signaling inhibitors including enzalutamide, abiraterone, apalutamide and darolutamide.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
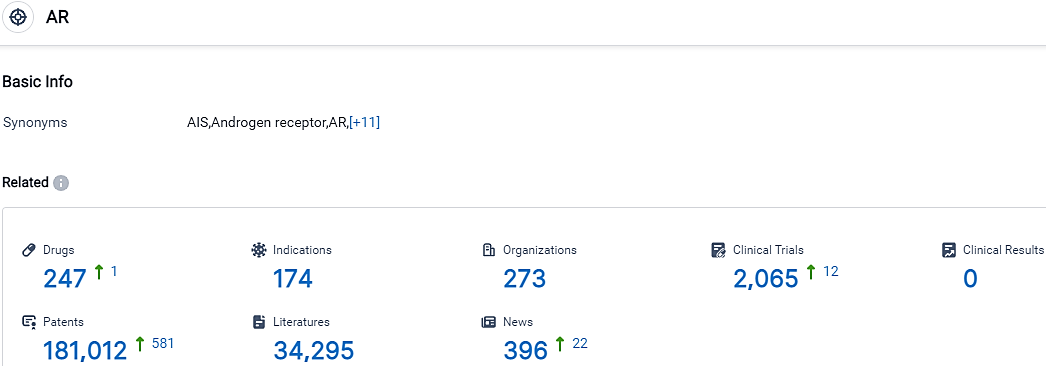
According to the data provided by the Synapse Database, As of October 17, 2023, there are 247 investigational drugs for the AR target, including 174 indications, 273 R&D institutions involved, with related clinical trials reaching 2065,and as many as 181012 patents.
ONCT-534 is categorized as a small molecule medication that primarily targets the androgen receptor. The medication has demonstrated potential in managing tumors, dermatological and musculoskeletal disorders, and urogenital ailments, with its current applications encompassing metastatic hormone resistant prostate cancer, castration-resistant prostate cancer, mammary cancer, and hormone resistant prostate cancer. Additional studies and testing in a clinical environment are required to affirm the effectiveness and risk profile of ONCT-534.