Analysis on the Research Progress of Neprilysin Inhibitor
Neprilysin, also known as membrane metallo-endopeptidase (MME), neutral endopeptidase (NEP), cluster of differentiation 10 (CD10), and common acute lymphoblastic leukemia antigen (CALLA) is an enzyme that in humans is encoded by the MME gene. Neprilysin is a zinc-dependent metalloprotease that cleaves peptides at the amino side of hydrophobic residues and inactivates several peptide hormones including glucagon, enkephalins, substance P, neurotensin, oxytocin, and bradykinin.[5] It also degrades the amyloid beta peptide whose abnormal folding and aggregation in neural tissue has been implicated as a cause of Alzheimer's disease. Synthesized as a membrane-bound protein, the neprilysin ectodomain is released into the extracellular domain after it has been transported from the Golgi apparatus to the cell surface.
These enzymes are responsible for breaking down enkephalins, which are endogenous opioid peptides involved in pain modulation and the regulation of emotions. By degrading enkephalins, enkephalinase enzymes help to control the duration and intensity of pain signals, as well as regulate mood and emotional responses. Understanding the role of enkephalinase in the human body is important for the development of pharmaceutical interventions targeting pain management and mood disorders.
Neprilysin Competitive Landscape
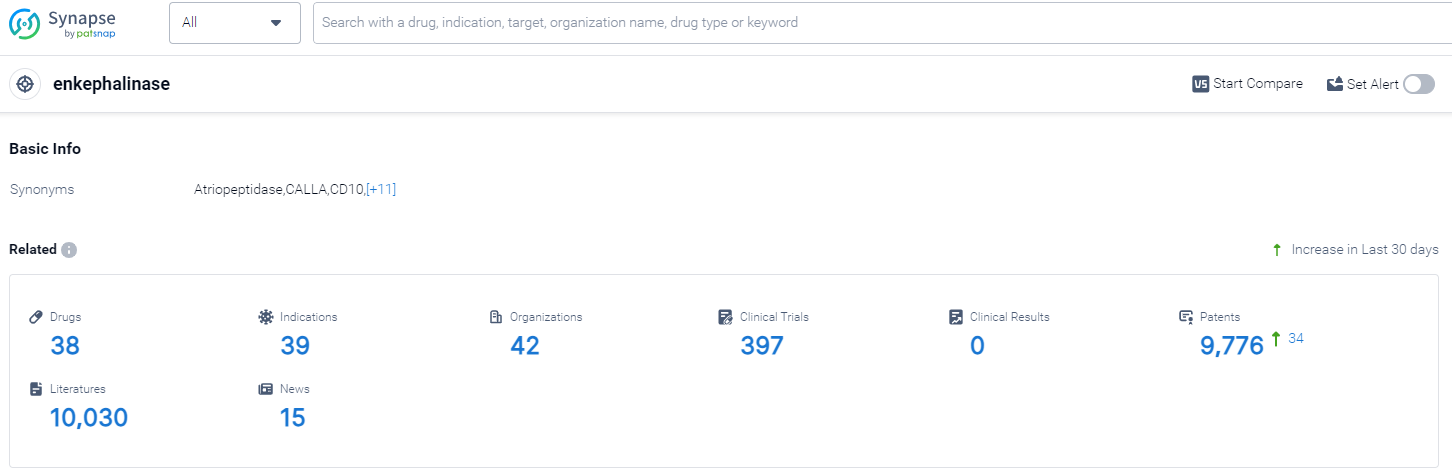
According to PatSnap Synapse, as of 13 Oct 2023, there are a total of 38 enkephalinase drugs worldwide, from 42 organizations, covering 39 indications, and conducting 397 clinical trials..
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the current competitive landscape of target enkephalinase reveals that Novartis AG is the leading company in terms of development stage, with multiple drugs in different phases. The indication analysis shows that drugs targeting enkephalinase have been approved for various indications, including hypertension, heart failure, and pain. The drug types progressing most rapidly are small molecule drugs and synthetic peptides, indicating intense competition in the development of innovative drugs. The country/location analysis highlights the European Union, China, and Argentina as regions with significant progress in the development of drugs targeting enkephalinase. Overall, the target enkephalinase presents a promising opportunity for pharmaceutical companies, with multiple companies and countries actively involved in research and development efforts.
Key drug:Racecadotril
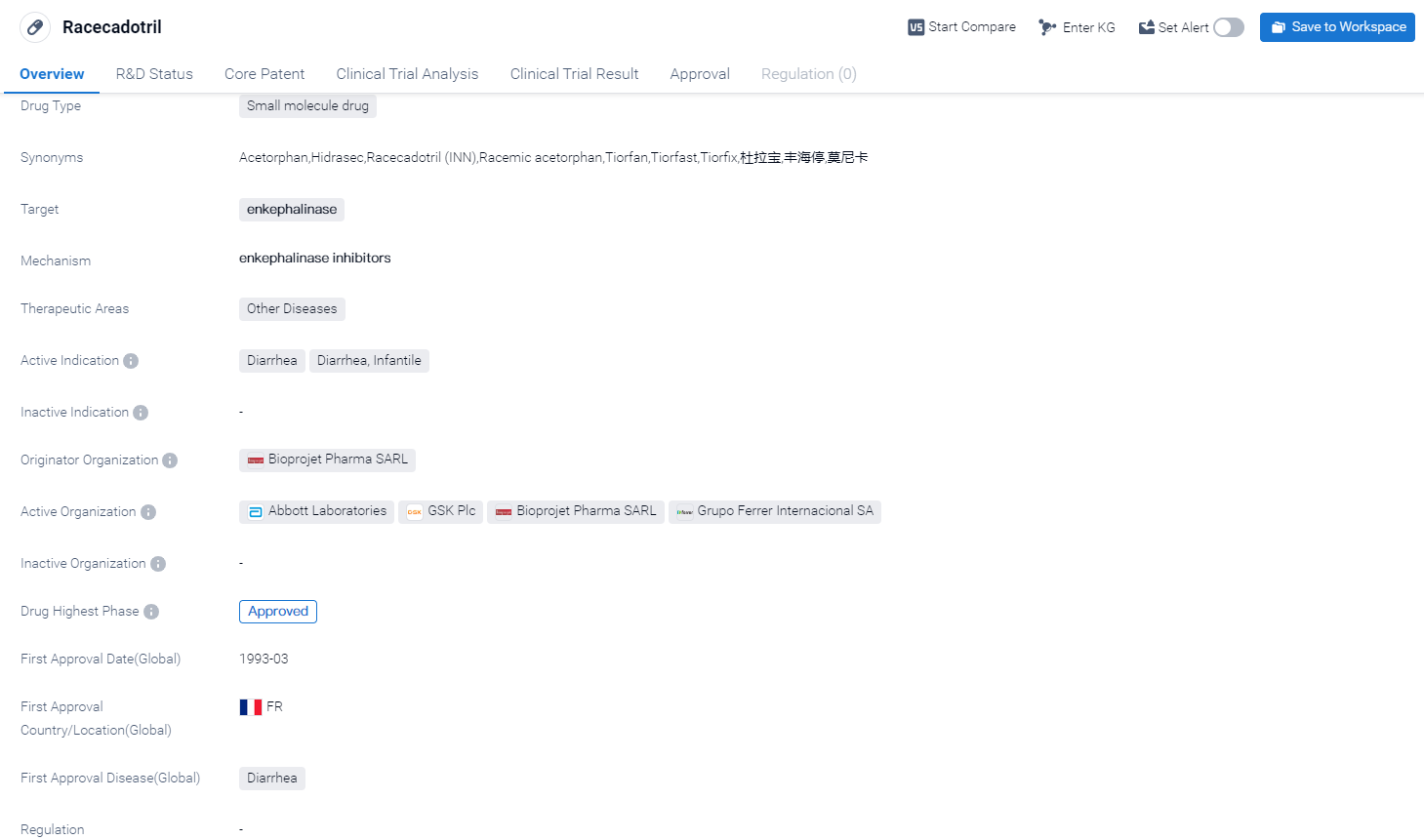
Racecadotril is a small molecule drug that targets enkephalinase and is primarily used in the treatment of diarrhea, specifically diarrhea in infants. It falls under the therapeutic area of Other Diseases. The drug was first approved in France in March 1993 and has since received approval in other countries as well.
Racecadotril works by inhibiting enkephalinase, an enzyme responsible for the degradation of enkephalins, which are natural opioids in the body. By inhibiting this enzyme, racecadotril helps to increase the levels of enkephalins, which in turn reduces the secretion of water and electrolytes in the intestines, leading to a decrease in diarrhea symptoms.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is primarily indicated for the treatment of diarrhea in infants, although it can also be used for diarrhea in adults. It is important to note that racecadotril is not a cure for diarrhea, but rather a symptomatic treatment that helps to alleviate the symptoms associated with the condition.
Racecadotril was developed by Bioprojet Pharma SARL, an originator organization in the pharmaceutical industry. It has reached the highest phase of approval in the global market, indicating its widespread acceptance and recognition as a safe and effective treatment option for diarrhea.
Since its approval in 1993, racecadotril has been used extensively in the management of diarrhea, particularly in infants. Its efficacy and safety profile have been well-established through clinical trials and real-world use, making it a trusted choice for healthcare professionals.
In summary, racecadotril is a small molecule drug that targets enkephalinase and is used for the treatment of diarrhea, specifically in infants. It was first approved in France in 1993 and has since gained approval in other countries. Developed by Bioprojet Pharma SARL, racecadotril has reached the highest phase of approval globally and in China, highlighting its significance in the pharmaceutical industry.