An In-depth Analysis of Tigecycline's R&D Progress and Mechanism of Action on Drug Target
Tigecycline's R&D Progress
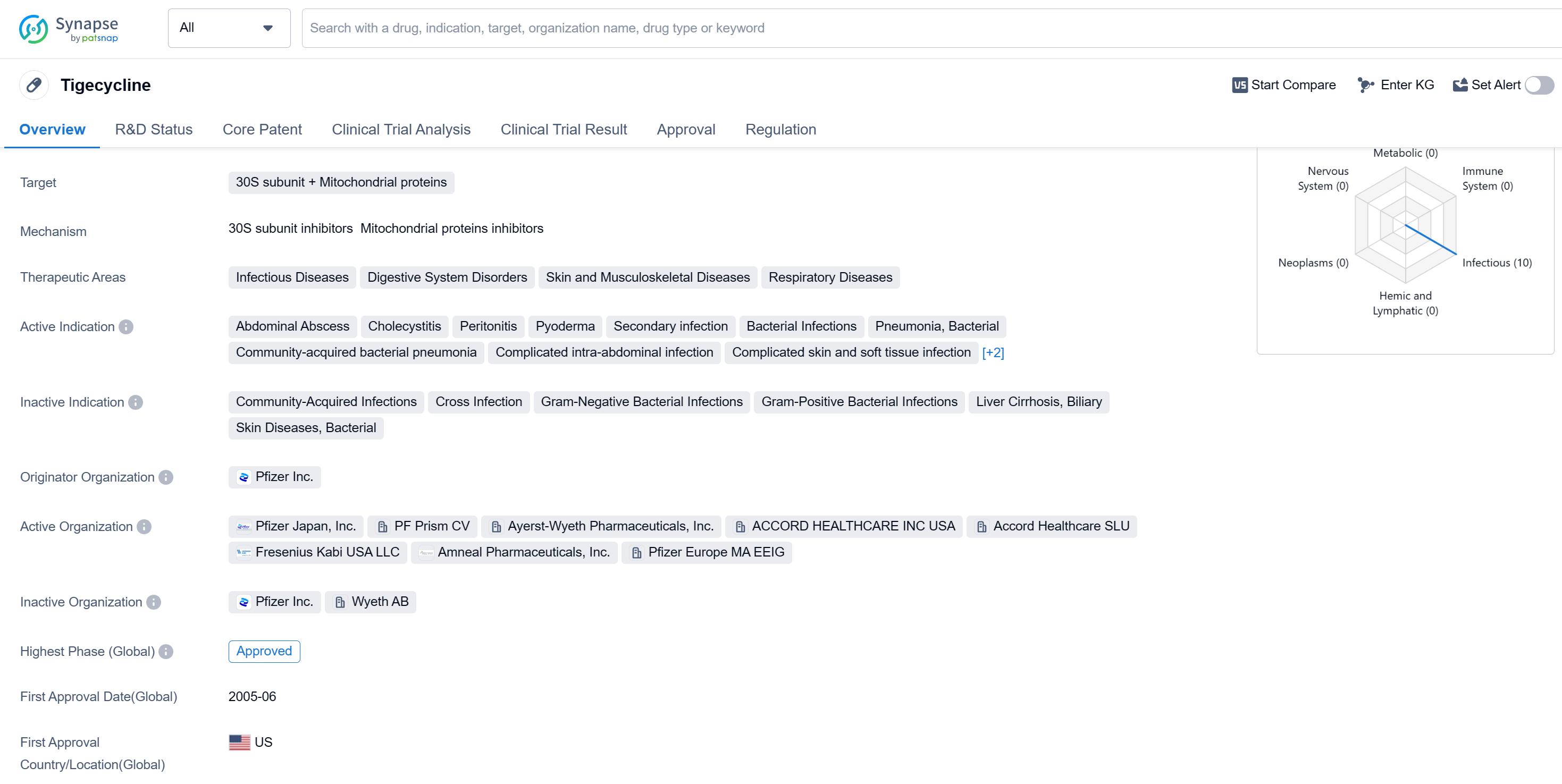
Tigecycline is a small molecule drug that targets the 30S subunit and mitochondrial proteins. It falls under the therapeutic areas of infectious diseases, digestive system disorders, skin and musculoskeletal diseases, and respiratory diseases. The drug is indicated for various conditions including abdominal abscess, cholecystitis, peritonitis, pyoderma, secondary infection, bacterial infections, pneumonia (bacterial and community-acquired), complicated intra-abdominal infection, complicated skin and soft tissue infection, and complicated skin and skin structure infection.
Tigecycline was first approved in the United States in June 2005 and is developed by Pfizer Inc., a renowned pharmaceutical company. Tigecycline has reached the highest phase of development which is approved globally. The drug is classified as an orphan drug, which suggests that it is intended to treat rare diseases or conditions.
As a small molecule drug, Tigecycline works by targeting the 30S subunit and mitochondrial proteins. This mechanism of action allows it to effectively combat bacterial infections and related complications. Its approval for various indications highlights its versatility in treating a range of diseases affecting different body systems.
The approval of Tigecycline in the United States and China signifies its compliance with regulatory standards and its potential to address unmet medical needs in these regions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tigecycline: 30S subunit inhibitors and Mitochondrial proteins inhibitors
30S subunit inhibitors are a type of drugs that specifically target the 30S subunit of bacterial ribosomes, which are involved in protein synthesis. These inhibitors prevent the binding of transfer RNA (tRNA) to the ribosome, thereby inhibiting the synthesis of bacterial proteins. By targeting the bacterial ribosomes, these inhibitors selectively inhibit bacterial protein synthesis without affecting the protein synthesis in human cells. This makes them effective antibiotics for treating bacterial infections.
Mitochondrial proteins inhibitors refer to drugs or compounds that target and inhibit specific proteins within the mitochondria, which are the powerhouses of the cell responsible for generating energy. These inhibitors can interfere with the normal functioning of mitochondrial proteins, disrupting various cellular processes that rely on mitochondrial function. By selectively inhibiting specific mitochondrial proteins, these inhibitors can be used to study the role of these proteins in cellular processes or as potential therapeutic agents for diseases associated with mitochondrial dysfunction, such as neurodegenerative disorders or metabolic diseases.
In summary, 30S subunit inhibitors are drugs that target bacterial ribosomes to inhibit protein synthesis, while mitochondrial proteins inhibitors are compounds that specifically inhibit certain proteins within the mitochondria, affecting cellular processes and potentially serving as therapeutic agents.
Drug Target R&D Trends for Tigecycline
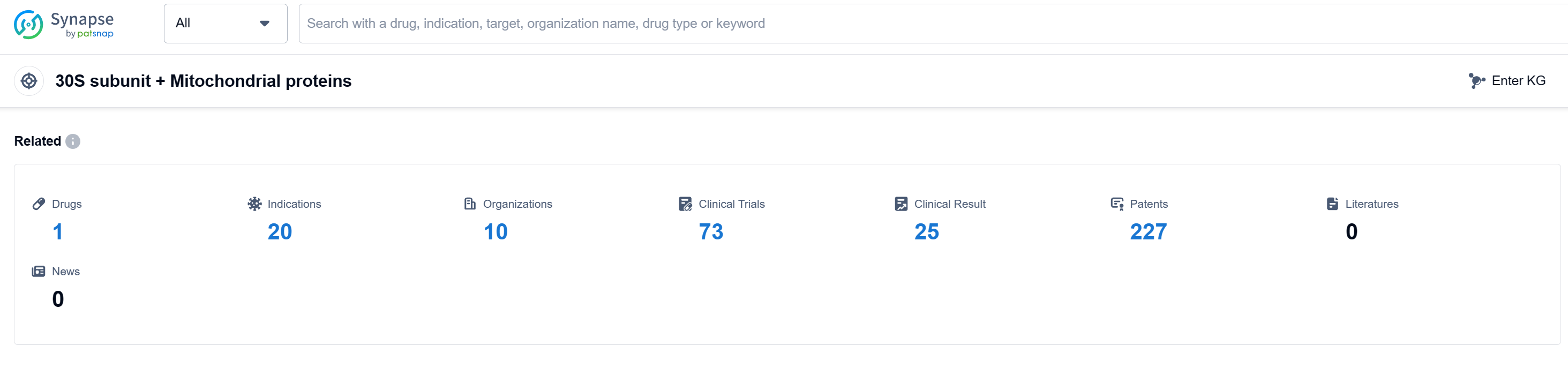
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 1 30S subunit + Mitochondrial proteins drugs worldwide, from 10 organizations, covering 20 indications, and conducting 73 clinical trials.
Based on the analysis of the provided data, Pfizer Inc. is the company with the highest number of drugs in the approved phase under the target 30S subunit + Mitochondrial proteins. The indications targeted by these drugs include various bacterial infections, pneumonia, intraabdominal infections, and skin diseases. The majority of drugs under this target are small molecule drugs. The countries/locations that have made progress in the development of drugs under this target include China, European Union, Japan, and the United States. China has also made progress in the development of drugs in the inactive phase. Overall, the competitive landscape for target 30S subunit + Mitochondrial proteins is characterized by the involvement of multiple companies, a focus on bacterial infections, and the dominance of small molecule drugs. The future development of this target will likely continue to be driven by these factors.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tigecycline is a small molecule drug developed by Pfizer Inc. It targets the 30S subunit and mitochondrial proteins, making it effective against various infectious diseases and related complications. Its approval in the United States and China, as well as its orphan drug status, further validate its potential in addressing unmet medical needs.