An In-depth Analysis of Xylometazoline Hydrochloride's R&D Progress
Xylometazoline Hydrochloride's R&D Progress
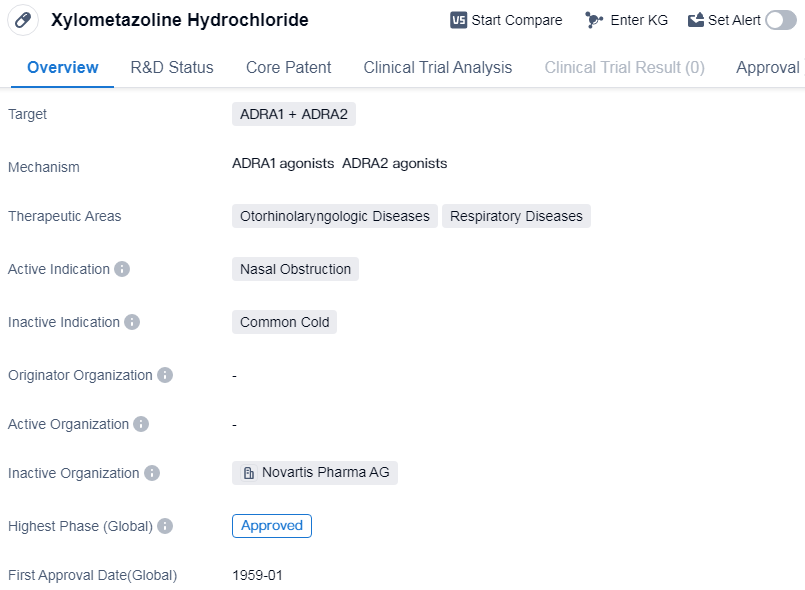
Xylometazoline Hydrochloride is a small molecule drug that targets ADRA1 and ADRA2 receptors. It is primarily used in the treatment of otorhinolaryngologic diseases and respiratory diseases, specifically for nasal obstruction. The drug has been approved in global markets.
Xylometazoline Hydrochloride has a long history in the pharmaceutical industry, with its first approval date in the global market dating back to January 1959. This indicates that the drug has been in use for several decades and has established a track record of safety and efficacy.
As a small molecule drug, Xylometazoline Hydrochloride is designed to interact with specific receptors in the body, namely ADRA1 and ADRA2. These receptors are involved in regulating nasal congestion and constriction of blood vessels. By targeting these receptors, Xylometazoline Hydrochloride helps to alleviate nasal obstruction, providing relief to individuals suffering from respiratory and otorhinolaryngologic diseases.
The therapeutic areas of otorhinolaryngologic diseases and respiratory diseases encompass a wide range of conditions, including sinusitis, rhinitis, and common cold. Nasal obstruction is a common symptom in these conditions, leading to difficulty in breathing and discomfort. Xylometazoline Hydrochloride offers a solution by reducing nasal congestion and improving airflow.
The fact that Xylometazoline Hydrochloride has reached the highest phase of approval in the global markets indicates its widespread acceptance and recognition by regulatory authorities. This suggests that the drug has undergone rigorous testing and evaluation to ensure its safety and efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Xylometazoline Hydrochloride: ADRA1 agonists and ADRA2 agonists
ADRA1 agonists and ADRA2 agonists are types of drugs that target specific receptors in the body known as adrenergic receptors. Adrenergic receptors are found on the surface of various cells and tissues and are involved in the response to the neurotransmitter norepinephrine.
ADRA1 agonists specifically activate the alpha-1 adrenergic receptors. These receptors are primarily located in smooth muscle tissues, such as blood vessels, and their activation leads to constriction or narrowing of the blood vessels. This can result in increased blood pressure and improved blood flow to certain areas of the body.
ADRA2 agonists, on the other hand, target the alpha-2 adrenergic receptors. These receptors are found in various regions of the central nervous system and peripheral tissues. Activation of ADRA2 receptors generally leads to inhibition of norepinephrine release, which can have various effects depending on the specific location of the receptors. In some cases, ADRA2 agonists can reduce sympathetic nervous system activity, resulting in decreased blood pressure and heart rate.
Both ADRA1 agonists and ADRA2 agonists have potential therapeutic applications. ADRA1 agonists are commonly used to treat conditions such as hypotension (low blood pressure), nasal congestion, and urinary retention. ADRA2 agonists can be used to manage conditions like hypertension (high blood pressure), attention deficit hyperactivity disorder (ADHD), and certain psychiatric disorders.
It's important to note that the use of these drugs should be done under the guidance of a healthcare professional, as they can have specific indications, contraindications, and potential side effects.
Drug Target R&D Trends for Xylometazoline Hydrochloride
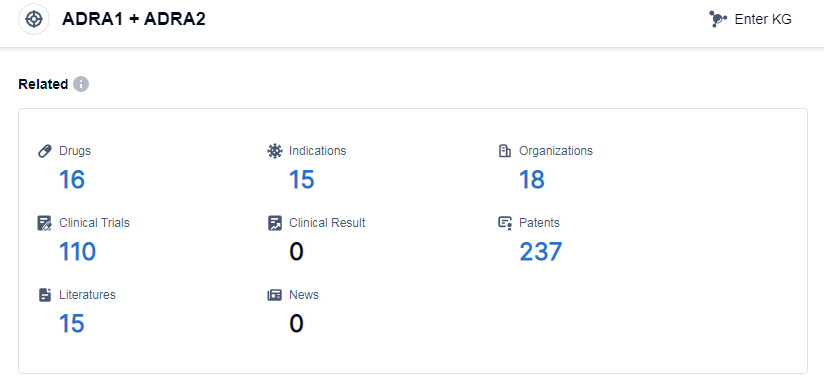
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 16 ADRA1 and ADRA2 drugs worldwide, from 18 organizations, covering 15 indications, and conducting 110 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for the target ADRA1 and ADRA2 is characterized by the presence of multiple companies at different stages of development. Merck & Co., Inc. is leading with one drug approved, followed by several other companies with one approved drug each. The indications for drugs under this target range from common cold to various allergic conditions, fever, and other indications. Small molecule drugs are progressing rapidly, indicating intense competition in the development of innovative drugs. China is emerging as a significant player in the development of drugs under this target, with 4 approved drugs. Overall, the target ADRA1 and ADRA2 shows promising development potential in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Xylometazoline Hydrochloride is a small molecule drug that targets ADRA1 and ADRA2 receptors. It is approved for the treatment of nasal obstruction in otorhinolaryngologic and respiratory diseases. With a long history of use since its first approval in 1959, Xylometazoline Hydrochloride has established itself as a reliable and effective option for individuals suffering from nasal congestion.