An Overview of Boehringer Ingelheim’s Pipeline|R&D Progress|Target
Boehringer Ingelheim GmbH is a pharmaceutical organization that was founded in 1885 and is headquartered in Rheinland-Pfalz, Germany. With a long history in the industry, Boehringer Ingelheim has established itself as a key player in the field of biomedicine. Boehringer-Ingelheim is a pharmaceutical company specializing in human bio-pharmaceutical chemistry and animal health products, and the world's largest unlisted private pharmaceutical company. Boehringer Ingelheim officially entered the Chinese market in 1994. Boehringer Ingelheim's main research areas include: immune and respiratory diseases, cardiovascular and metabolic diseases, central nervous system diseases, and oncology. The core business is including prescription drugs, animal health and biopharmaceuticals. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of drugs for Boehringer Ingelheim.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Boehringer Ingelheim.
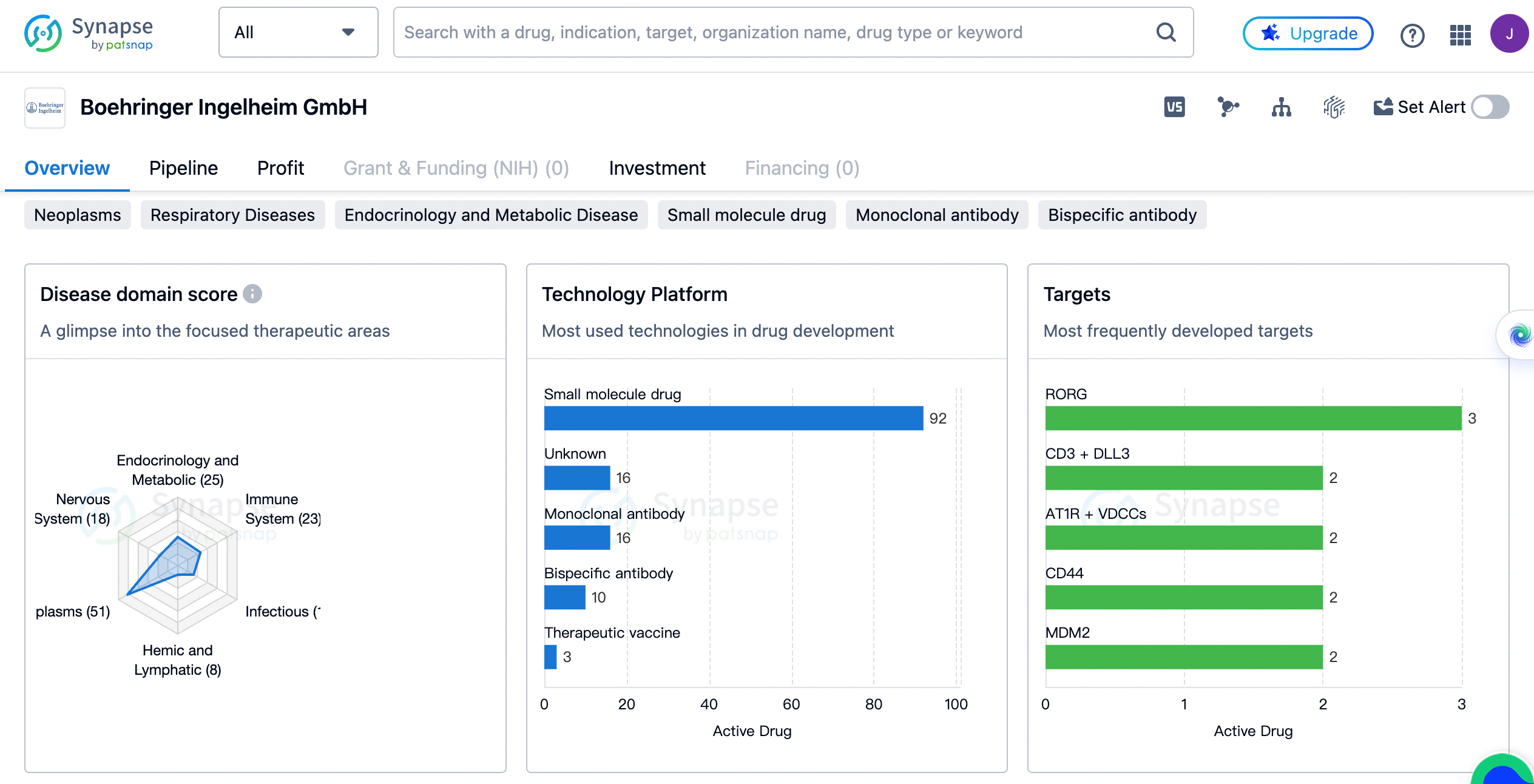
The distribution of therapeutic areas
Firstly, let's examine the distribution of therapeutic areas and the corresponding drug count for Boehringer Ingelheim. The organization has a diverse portfolio of drugs that cater to various medical needs. The therapeutic area with the highest number of drugs is Neoplasms, with a count of 51. This indicates that Boehringer Ingelheim has a strong focus on developing treatments for cancer patients. Respiratory Diseases is the second most prominent therapeutic area, with 42 drugs. This suggests that the organization is actively involved in addressing respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD).
Digestive System Disorders and Endocrinology and Metabolic Disease follow closely with 27 and 25 drugs, respectively. These numbers indicate that Boehringer Ingelheim is committed to developing treatments for gastrointestinal and metabolic disorders. Immune System Diseases, Other Diseases, Cardiovascular Diseases, Nervous System Diseases, and Skin and Musculoskeletal Diseases are also areas of focus for the organization, with drug counts ranging from 23 to 17. Infectious Diseases, Urogenital Diseases, Eye Diseases, Congenital Disorders, Hemic and Lymphatic Diseases, Mouth and Tooth Diseases, and Otorhinolaryngologic Diseases have relatively lower drug counts, ranging from 16 to 2. This distribution of therapeutic areas showcases Boehringer Ingelheim's commitment to addressing a wide range of medical conditions.
The most frequently developed targets by boehringer ingelheim
Next, let's explore the most frequently developed targets by Boehringer Ingelheim. The organization has invested in the development of drugs targeting various proteins and receptors. RORG is the most frequently developed target, with a count of 3 drugs. CD3 + DLL3, AT1R + VDCCs, CD44, MDM2, GABAA receptor, β2-adrenergic receptor, CFTR, B1 receptor, ALDOS, PLG, and FAK are also targets that have been developed by Boehringer Ingelheim, with a count of 2 drugs each. These targets represent a diverse range of proteins and receptors involved in different disease pathways. Additionally, there are several targets with a count of 1 drug, indicating that Boehringer Ingelheim has explored a wide range of targets in its drug development efforts.
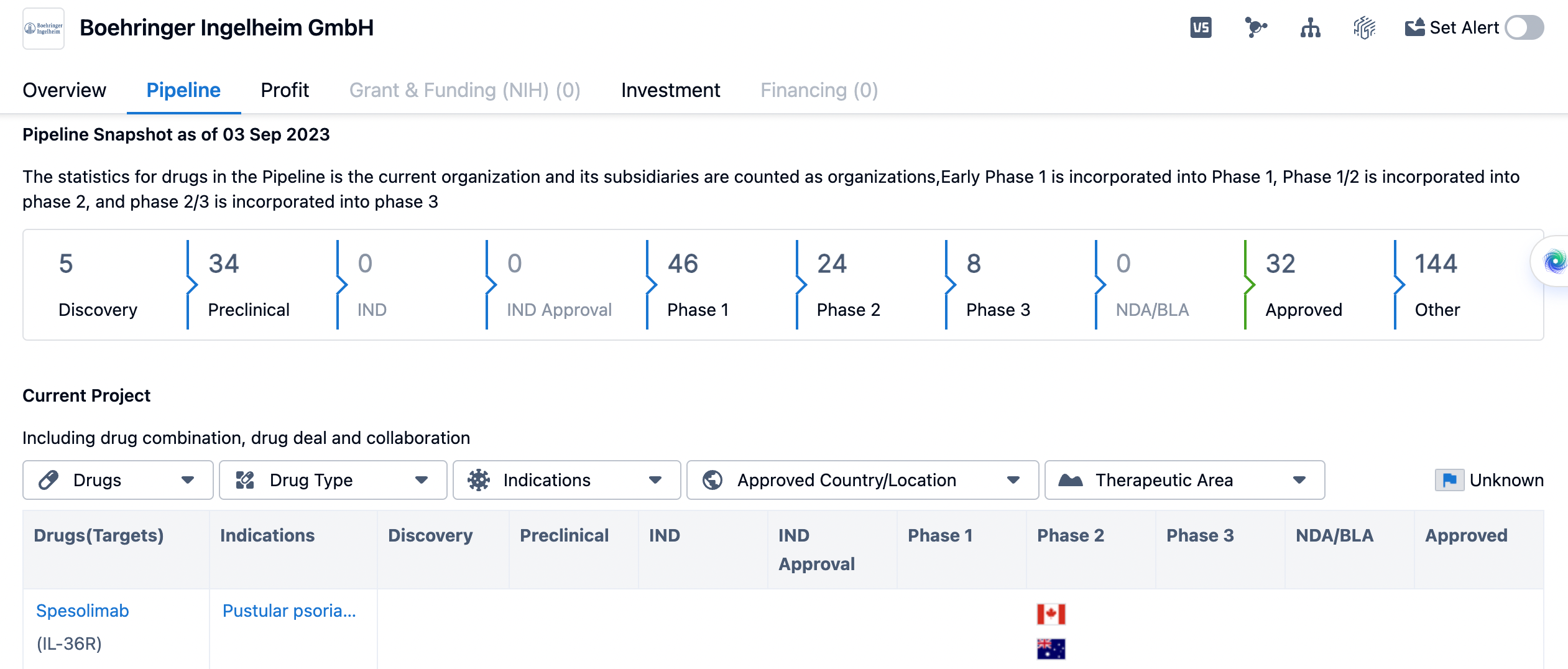
The pipeline of drugs for boehringer ingelheim
Lastly, let's analyze the pipeline of drugs for Boehringer Ingelheim. The pipeline provides insights into the various stages of drug development and the number of drugs in each phase. Boehringer Ingelheim has 5 drugs in the Discovery phase, indicating ongoing research and early-stage development. The Preclinical phase has the highest number of drugs, with 34 in the pipeline. This suggests that Boehringer Ingelheim has a robust pipeline of drugs undergoing preclinical testing and evaluation. In the subsequent phases, Boehringer Ingelheim has 46 drugs in Phase 1, 24 drugs in Phase 2, and 8 drugs in Phase 3. These numbers indicate that the organization has a significant number of drugs progressing through clinical trials. In terms of regulatory approval, there are no drugs in the NDA/BLA phase, but 32 drugs have already been approved. Additionally, there are 144 drugs categorized as "Other" in the pipeline, which could include drugs in various stages of development or those with uncertain timelines.
In summary, Boehringer Ingelheim GmbH is a pharmaceutical organization with a rich history in the industry. The distribution of therapeutic areas highlights the organization's commitment to addressing a wide range of medical conditions, with a strong focus on Neoplasms and Respiratory Diseases. The most frequently developed targets indicate a diverse range of proteins and receptors that Boehringer Ingelheim has targeted in its drug development efforts. The pipeline analysis reveals a significant number of drugs in preclinical and clinical stages, demonstrating the organization's ongoing commitment to research and development. Overall, Boehringer Ingelheim's extensive portfolio and pipeline reflect its dedication to advancing biomedicine and improving patient outcomes.