An Overview of Fresenius 's 51 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
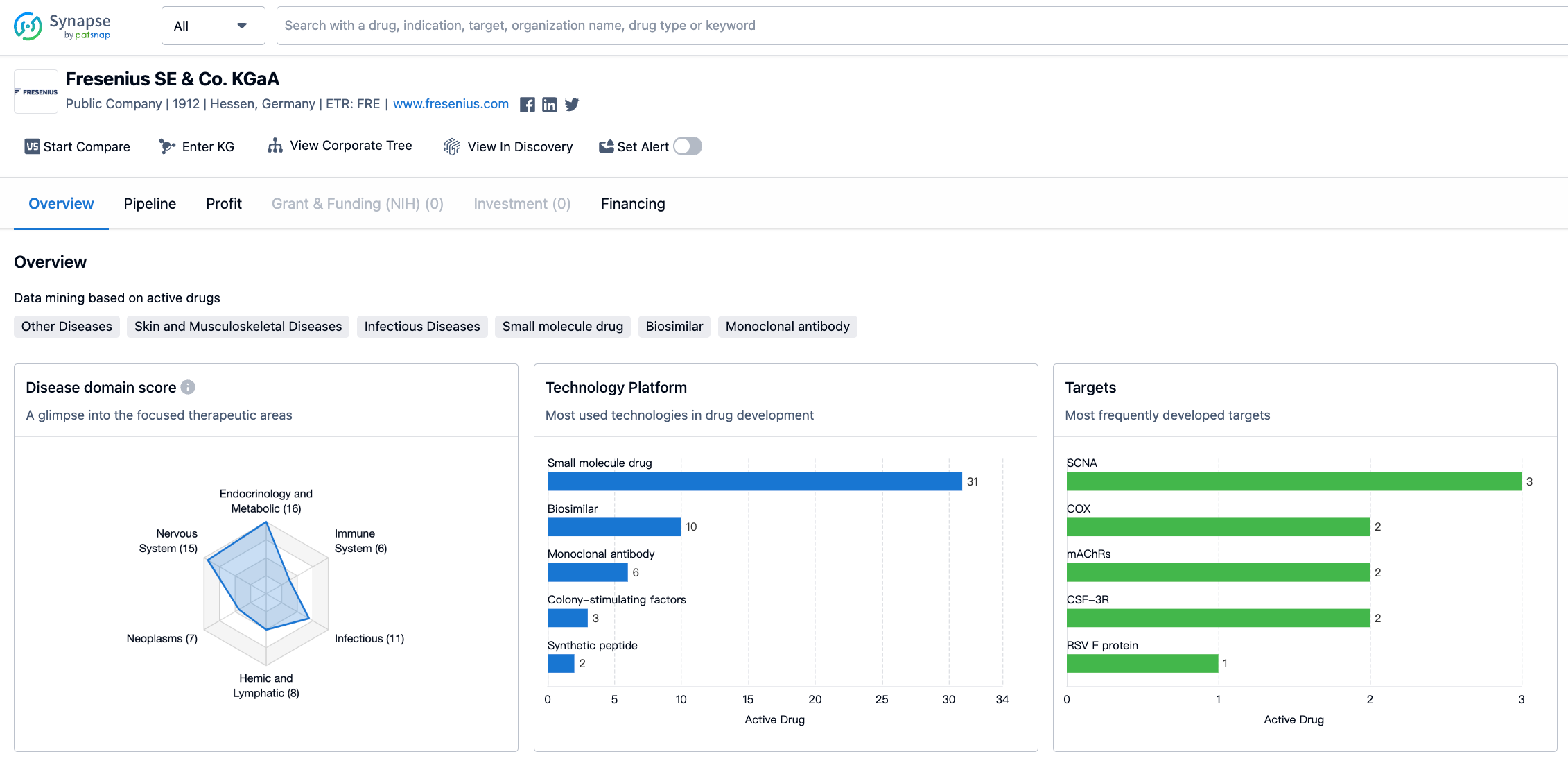
Fresenius SE & Co. KGaA is a pharmaceutical organization that was founded in 1912 and is based in Hessen, Germany. The company has a diverse portfolio of drugs that target various therapeutic areas. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of drugs for Fresenius SE & Co. KGaA.
The organization has developed drugs for a wide range of therapeutic areas, including Other Diseases, Endocrinology and Metabolic Disease, Nervous System Diseases, Skin and Musculoskeletal Diseases, Respiratory Diseases, Infectious Diseases, Digestive System Disorders, Urogenital Diseases, Cardiovascular Diseases, Hemic and Lymphatic Diseases, Neoplasms, Congenital Disorders, Immune System Diseases, Eye Diseases, Mouth and Tooth Diseases, and Otorhinolaryngologic Diseases. The highest drug count is for Other Diseases, with 17 drugs, followed closely by Endocrinology and Metabolic Disease with 16 drugs. The lowest drug count is for Otorhinolaryngologic Diseases, with only 1 drug.
Fresenius SE & Co. KGaA. has focused on developing drugs that target a variety of proteins and receptors, including SCNA, COX, mAChRs, CSF-3R, RSV F protein, LHCGR, VEGF-A, IL-6RA, thrombin, THR, ER, Sodium channels, TNF-α, SCNA + adrenergic receptor, CD20, 1,3-beta-glucan synthase, AChE, RANKL, Phosphates, and GABAA receptor. The most frequently developed target is SCNA, with 3 drugs, followed by COX and mAChRs, each with 2 drugs. The remaining targets have been the focus of development for 1 drug each.
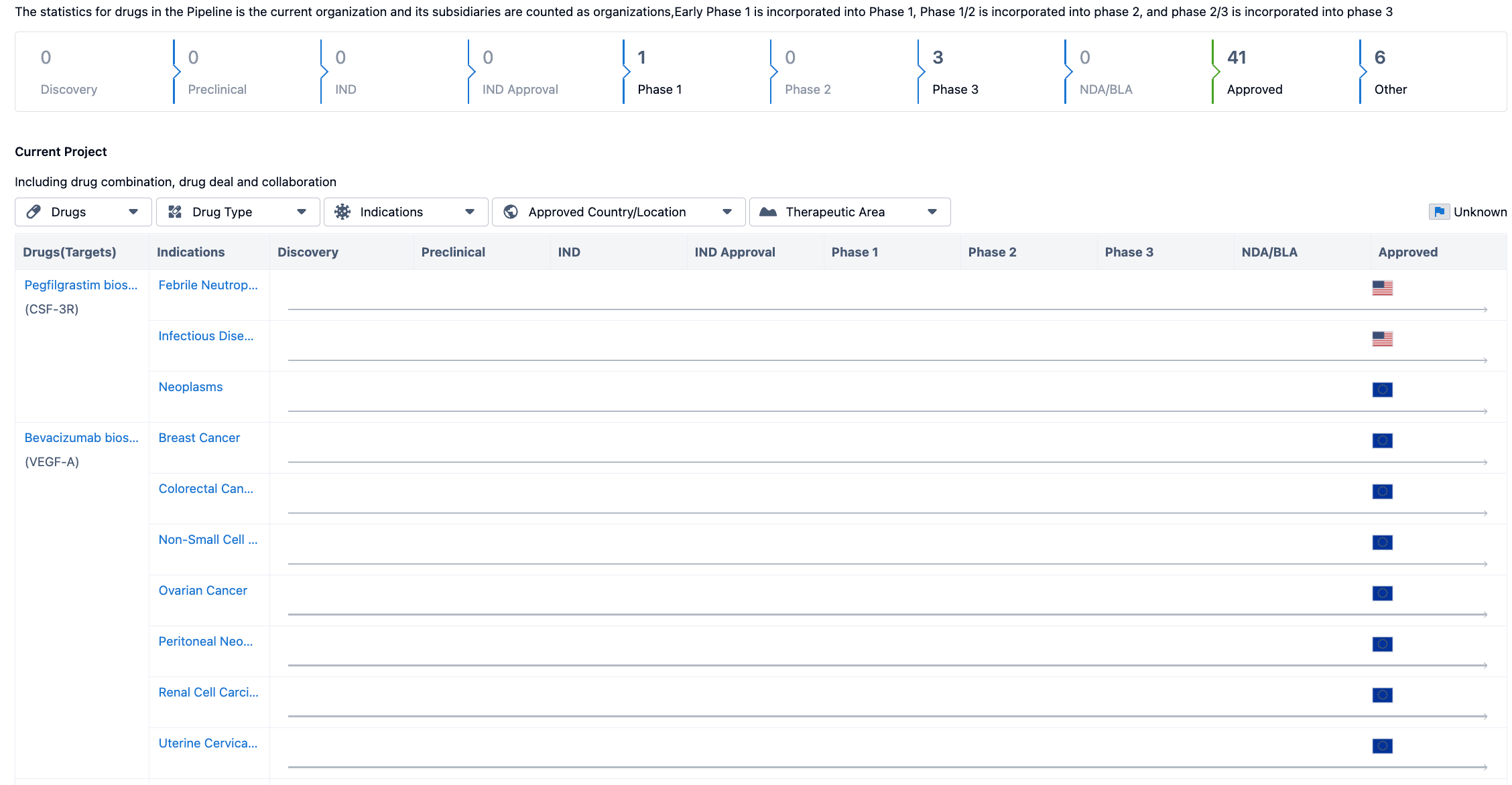
The pipeline is categorized into different phases, including Discovery, Preclinical, IND, IND Approval, Phase 1, Phase 2, Phase 3, NDA/BLA, Approved, and Other. As of the given date, there are no drugs in the Discovery, Preclinical, IND, or IND Approval phases. However, there is 1 drug in Phase 1, indicating that it is currently undergoing initial clinical trials. There are no drugs in Phase 2, but there are 3 drugs in Phase 3, suggesting that they are in advanced stages of clinical development. It is important to note that there are no drugs in the NDA/BLA phase, indicating that no drugs have been submitted for regulatory approval. However, there are 41 drugs that have been approved, indicating that they have successfully completed the regulatory process. Additionally, there are 6 drugs categorized as Other, which could include drugs in various stages of development or those that do not fit into the defined pipeline phases.
In summary, Fresenius SE & Co. KGaA is a pharmaceutical organization with a long history in the industry. The company has developed drugs for a wide range of therapeutic areas, with a focus on Other Diseases and Endocrinology and Metabolic Disease. They have also targeted various proteins and receptors, with SCNA being the most frequently developed target. The drug pipeline for Fresenius SE & Co. KGaA indicates that they have drugs in different stages of development, with a significant number of drugs already approved. This information provides valuable insights into the organization's activities and areas of focus in the pharmaceutical industry.