An Overview of Sumitomo Dainippon's 304 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
Sumitomo Pharma Co., Ltd. is a pharmaceutical organization based in Osaka-fu, Japan. It was founded in 1984 and has since been actively involved in the field of biomedicine. The company has a diverse portfolio of drugs targeting various therapeutic areas.
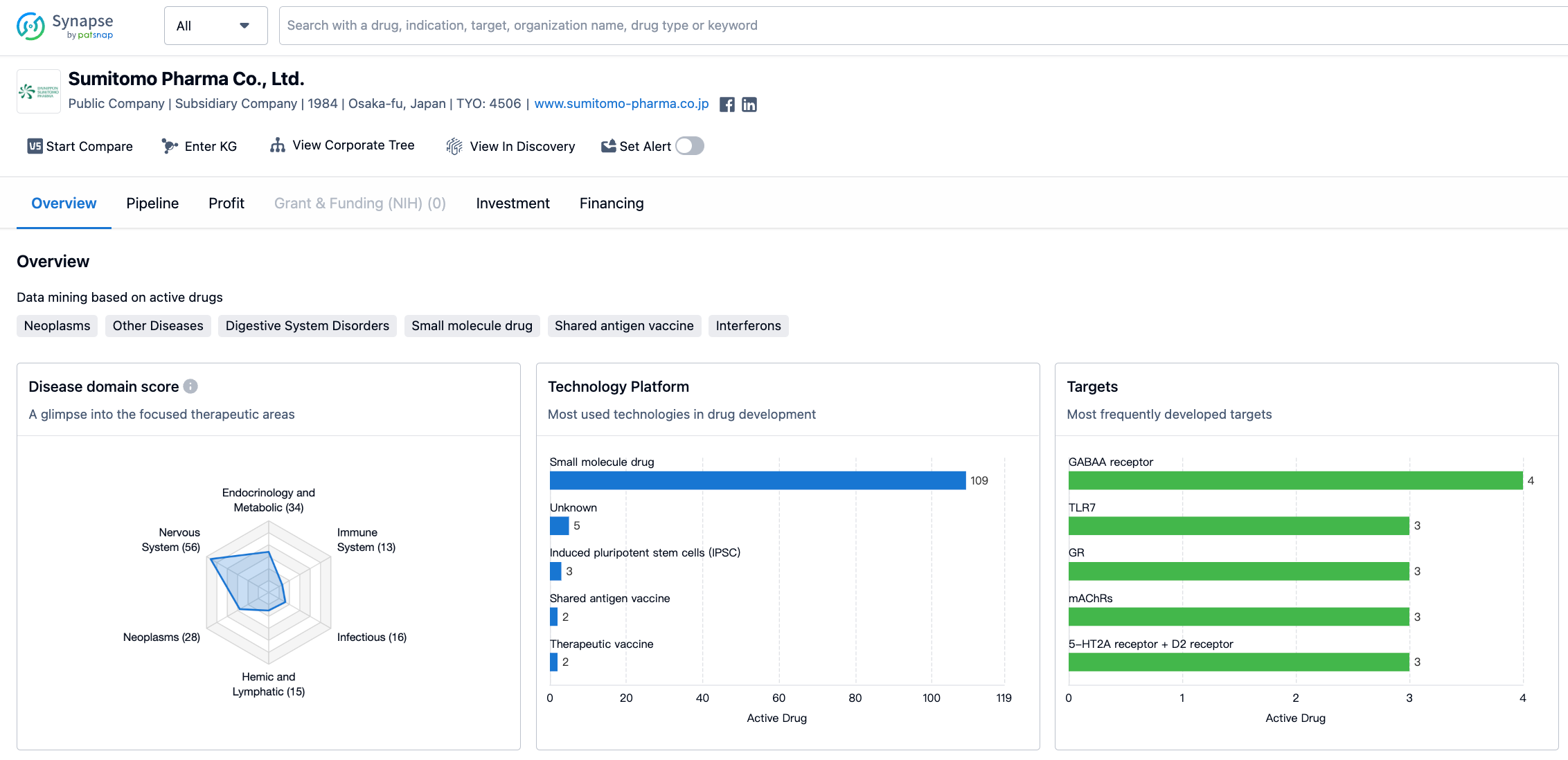
Sumitomo Pharma Co., Ltd. has developed drugs for a wide range of therapeutic areas. The highest number of drugs, 56 in total, are focused on Nervous System Diseases. This indicates that the company has a strong emphasis on neurological disorders and related conditions. Other Diseases and Endocrinology and Metabolic Disease follow closely with 41 and 34 drugs respectively. Neoplasms, Urogenital Diseases, and Cardiovascular Diseases are also areas of focus for the company.
In terms of drug development targets, Sumitomo Pharma Co., Ltd. has concentrated on several key targets. The GABAA receptor is the most frequently developed target, with 4 drugs in its portfolio. TLR7, GR, mAChRs, and the combination of 5-HT2A receptor and D2 receptor are also important targets with 3 drugs each. This suggests that the company is actively exploring these targets for potential therapeutic interventions. Other targets such as STAT3, β2-adrenergic receptor, WT1, DRDs, and 5-HT1A receptor have also been pursued by the company.
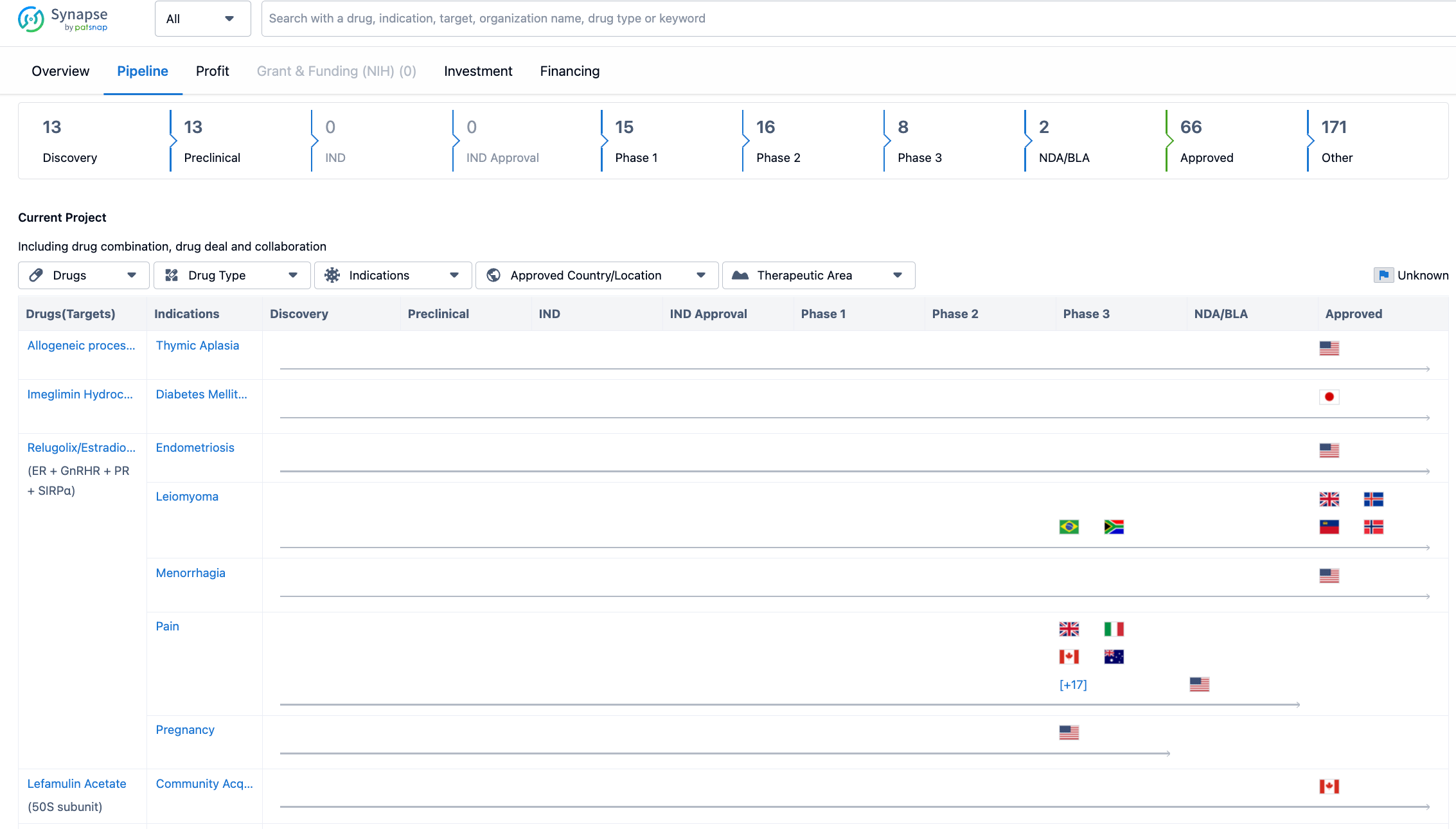
Looking at the pipeline of Sumitomo Pharma Co., Ltd. till 2023-07-24, the company has a significant number of drugs in various stages of development. The majority of drugs, 171 in total, are in the "Other" category, indicating that they are in early stages of development or have not yet progressed to specific phases. This suggests that the company has a robust pipeline and is actively exploring new drug candidates.
In terms of specific phases, the company has 13 drugs in the Discovery and Preclinical stages, indicating ongoing research and development efforts. However, there are no drugs in the IND (Investigational New Drug) or IND Approval stages, suggesting that the company may not have reached the stage of seeking regulatory approval for clinical trials. The Phase 1 stage has 15 drugs, followed by Phase 2 with 16 drugs and Phase 3 with 8 drugs. This indicates that the company has a significant number of drugs in advanced stages of clinical development. The NDA/BLA (New Drug Application/Biologics License Application) stage has 2 drugs, suggesting that Sumitomo Pharma Co., Ltd. has successfully completed clinical trials and is seeking regulatory approval for these drugs. Finally, the Approved stage has 66 drugs, indicating that the company has a substantial number of drugs that have received regulatory approval and are available in the market.
In summary, Sumitomo Pharma Co., Ltd. is a pharmaceutical organization with a strong focus on the development of drugs for various therapeutic areas. The company has a significant number of drugs targeting Nervous System Diseases, Other Diseases, and Endocrinology and Metabolic Disease. It has also explored a range of targets, with a particular emphasis on the GABAA receptor, TLR7, GR, mAChRs, and the combination of 5-HT2A receptor and D2 receptor. The company has a robust pipeline, with drugs in various stages of development, including Discovery, Preclinical, Phase 1, Phase 2, Phase 3, and Approved. This indicates that Sumitomo Pharma Co., Ltd. is actively engaged in research and development and has a strong presence in the pharmaceutical industry.