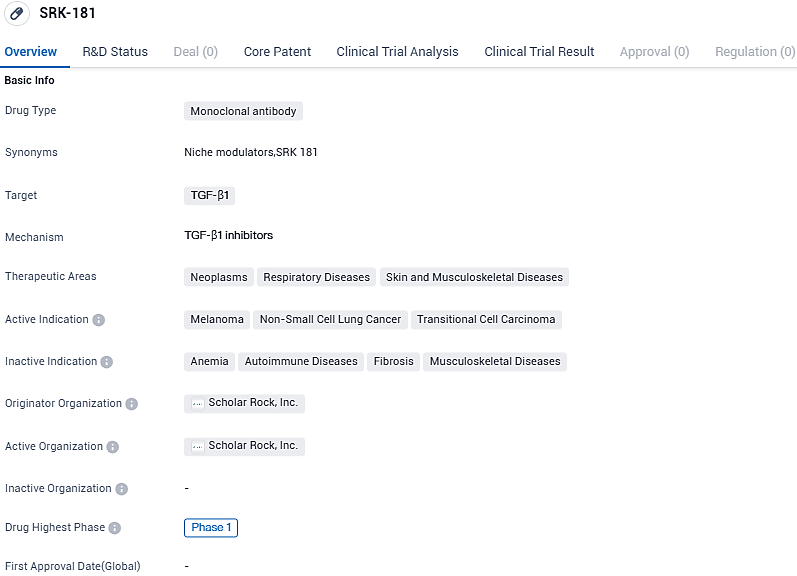
Scholar Rock reports promising anti-tumor results from phase 1 DRAGON trial for resistant metastatic ccRCC patients. Data supports continued tolerability of SRK-181
Scholar Rock, which is a biopharmaceutical business in its Phase 3 clinical-stage phase, concentrates on treating severe illnesses where protein growth factors have a crucial role. The company has declared fresh findings from its SRK-181 Phase 1 DRAGON proof-of-concept study, a designated latent TGFβ1 activation blocker, which is under development with the goal of bypassing resistance to checkpoint inhibitor therapy among patients suffering from advanced cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The primary poster accentuates the safety, effectiveness, and early biomarker data in patients affected by clear cell renal cell carcinoma resistant to anti-PD-1, within both A2 part (dose increase) and B part (dose enlargement) of Phase 1 DRAGON study. The ccRCC group was the emphasis of this poster having been the quickest group to meet enrollment objectives. The secondary poster emphasizes on the early biomarker data from B part of the examination in patients with several tumor kinds.
The reported data continues to validate the theory for SRK-181 in 28 largely prior treated patients with ccRCC resistant to anti-PD-1. SRK-181 was generally tolerated and demonstrated prospective anti-tumor mechanism in this group of patients. Six out of 28 assessable patients in the ccRCC group, who were treated with SRK-181 along with pembrolizumab, had confirmed partial responses and managed to reach a top tumor decrease of 33% to 93%, with a goal response rate of 21.4%.
"The DRAGON study has effectively met its declared objective of showcasing evidence of theory for SRK-181 by indicating prospective anti-tumor mechanism. The results, along with biomarker outcomes that validate the theory of the mechanism, brings to light the immunosuppressive role of TGFβ as a mechanism of anti-PD-1 resistance in patients” stated Jay Backstrom, M.D., M.P.H., President and CEO of Scholar Rock. "We are especially inspired by the responses seen in patients with ccRCC who had been treated with several therapy lines before getting SRK-181."
SRK-181 is a selective inhibitor of TGFβ1 activation developed to overcome inherent resistance to checkpoint inhibitor therapy, such as anti-PD-(L)1 antibodies, in advanced cancer cases. Data derived from various human tumors that are resistant to anti-PD-(L)1 therapy, imply that TGFβ1 is a significant contributor to the immunosuppressive tumor microenvironment, barring and hindering entry of cytotoxic T cells into the tumor, thus inhibiting anti-tumor immunity.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
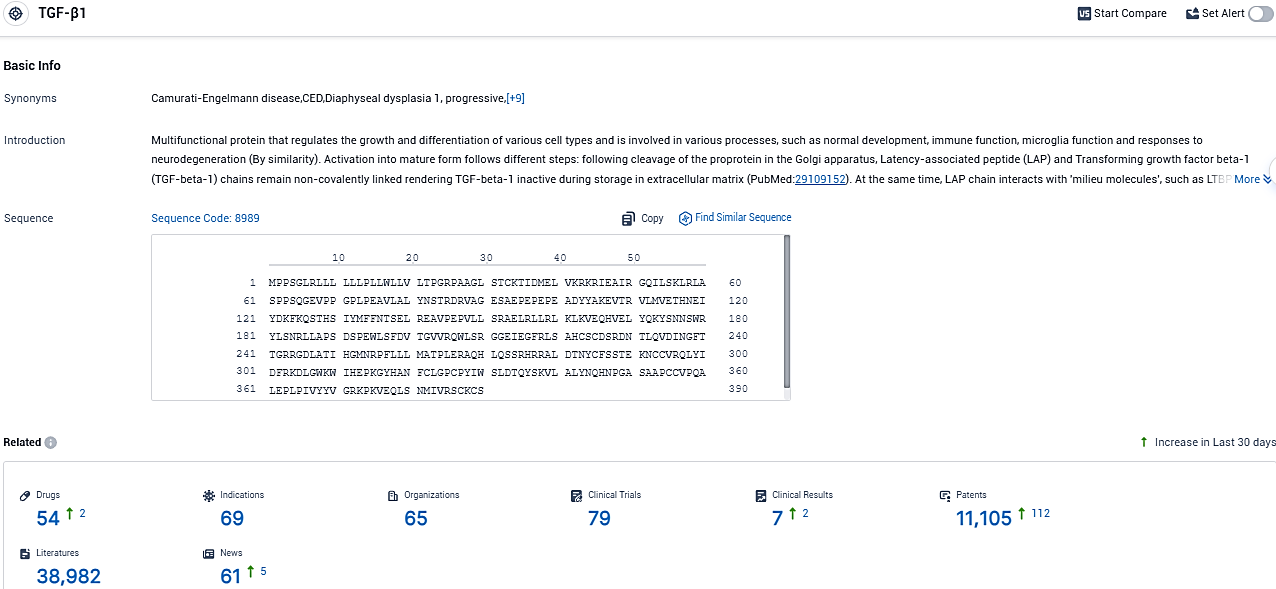
According to the data provided by the Synapse Database, As of November 11, 2023, there are 54 investigational drugs for the TGFβ1 target, including 69 indications, 65 R&D institutions involved, with related clinical trials reaching 79, and as many as 11105 patents.
SRK-181 has the potential to overcome this immune cell exclusion and induce tumor regression when administered in combination with anti-PD-(L)1 therapy while potentially avoiding toxicities associated with non-selective TGFβ inhibition. SRK-181 is an investigational product candidate and its efficacy and safety have not been established. SRK-181 has not been approved for any use by the FDA or any other regulatory agency.