Analysis on the Clinical Research Progress of Menin Inhibitor
Menin is an expression product of the tumorigenic gene MEN1 in multiple endocrine neoplasia. Multiple endocrine neoplasia syndrome is a familial autosomal dominant disease characterized by endocrine organ tumors such as thyroid, pancreatic islets, and anterior pituitary tumors. The occurrence of facial vascular fibroma, glioma, and lipomas are also related to MEN1.
Studies show that abnormal Menin expression is associated with the occurrence of diseases such as leukemia and diabetes. The mechanism by which mutations promote tumor formation is mainly through the loss of function of the two alleles, causing minor mutations such as missense mutations, or larger mutations such as heterozygous loss. More than 400 types of MEN1 mutations have been found in mammals and microorganisms, most of which are nonsense mutations and frameshift mutations. These two types of mutations lead to shortened gene encoding or inactivation of Menin, thereby changing the key function of Menin in inhibiting tumor activity. In pancreatic islet cells, Menin mutations will lead to excessive proliferation of β cells and result in islet β-cell tumors. Excessive Menin expression levels can obstruct the proliferation process of β cells, leading to relatively insufficient insulin secretion.
Menin is a protein encoded by the MEN1 gene and plays a crucial role in the human body. It acts as a tumor suppressor and is primarily found in the nucleus of cells. Menin is involved in regulating gene expression, cell division, and DNA repair processes. It interacts with various proteins and transcription factors to control cell growth and differentiation. Mutations in the MEN1 gene can lead to the development of multiple endocrine neoplasia type 1 (MEN1) syndrome, a hereditary disorder characterized by the formation of tumors in various endocrine organs. Understanding the role of menin is essential for developing targeted therapies for MEN1 syndrome and other related conditions.
MENIN Competitive Landscape
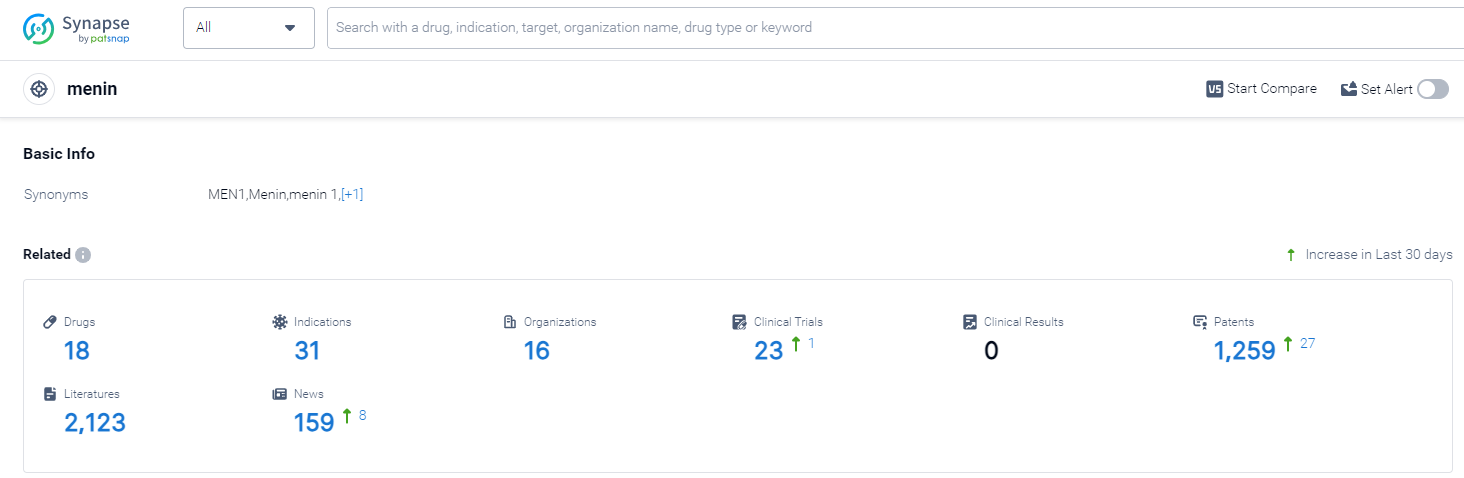
According to Patsnap Synapse, as of 13 Oct 2023, there are a total of 18 menin drugs worldwide, from 16 organizations, covering 31 indications, and conducting 23 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target menin in the pharmaceutical industry reveals a competitive landscape with multiple companies actively pursuing R&D in this area. The highest stage of development is Phase 2, indicating promising progress in the development of drugs targeting menin. Indications such as various types of leukemia, diabetes mellitus type 2, solid tumors, and lymphomas highlight the potential therapeutic applications of these drugs. Small molecule drugs and chemical drugs are progressing rapidly, indicating intense competition and the need for further analysis of biosimilars. The United States, China, and several European countries are leading in terms of R&D progress, with China showing significant progress in recent years. Overall, the target menin presents a promising area for future development in the pharmaceutical industry.
Key drug: BMF-219
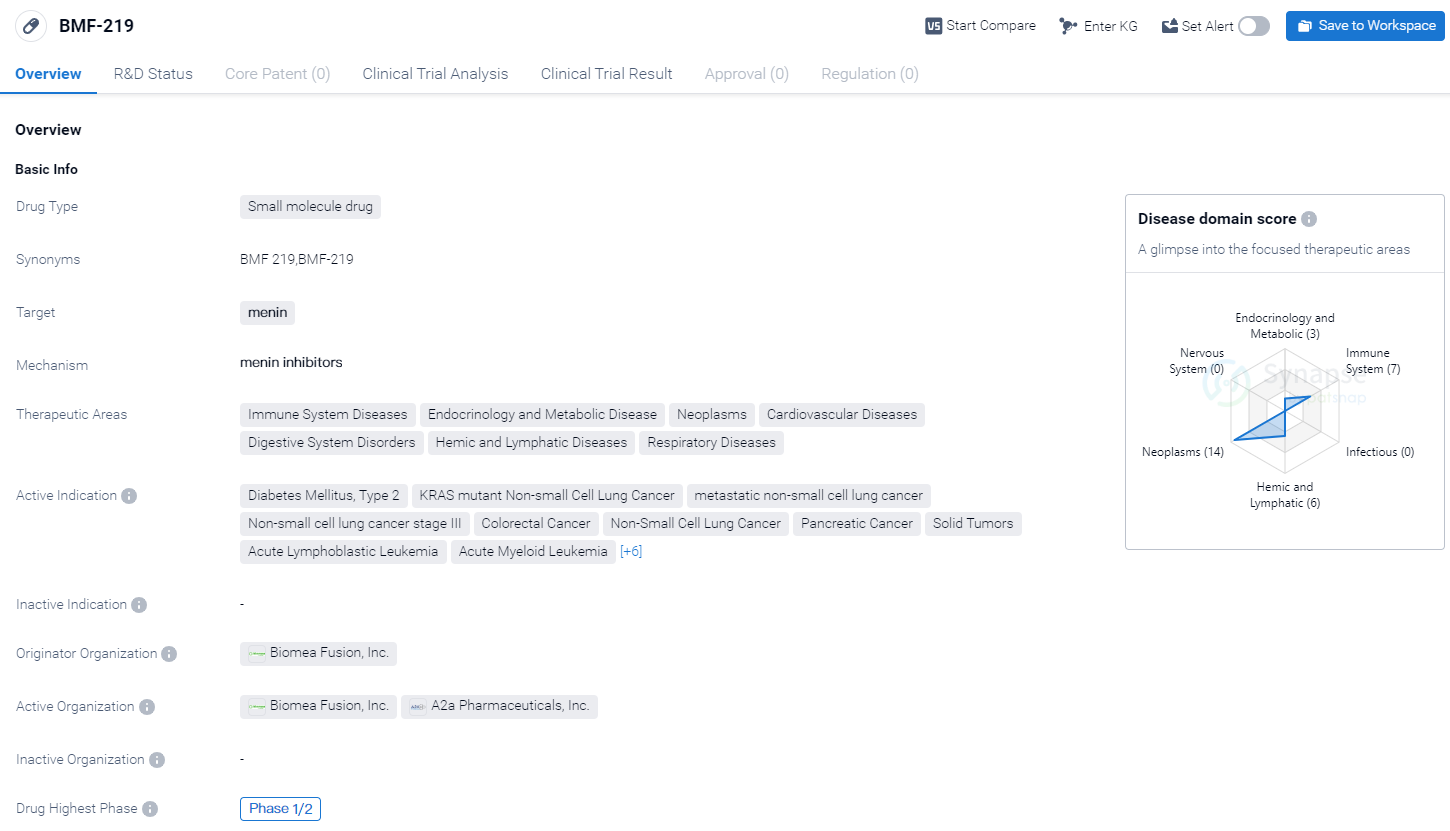
BMF-219 is a small molecule drug developed by Biomea Fusion, Inc. It is designed to target menin, a protein involved in various cellular processes. The drug is currently in the highest phase of clinical development, Phase 1/2, indicating that it has progressed beyond preclinical studies and is being tested in human subjects.
The therapeutic areas that BMF-219 aims to address are diverse, spanning across Immune System Diseases, Endocrinology and Metabolic Disease, Neoplasms, Cardiovascular Diseases, Digestive System Disorders, Hemic and Lymphatic Diseases, and Respiratory Diseases. This suggests that the drug has the potential to be used in the treatment of a wide range of conditions.
The active indications for BMF-219 include Diabetes Mellitus, Type 2, KRAS mutant Non-small Cell Lung Cancer, metastatic non-small cell lung cancer, Non-small cell lung cancer stage III, Colorectal Cancer, Non-Small Cell Lung Cancer, Pancreatic Cancer, Solid Tumors, Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, B-Cell Chronic Lymphocytic Leukemia, Diffuse Large B-Cell Lymphoma, Multiple Myeloma, Non-Hodgkin Lymphoma, Small Lymphocytic Lymphoma, and Diabetes Mellitus, Type 1. This extensive list suggests that BMF-219 has the potential to be a broad-spectrum therapeutic agent.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As the drug is currently in Phase 1/2, it is still undergoing clinical trials to evaluate its safety, efficacy, and optimal dosage. Phase 1/2 trials typically involve a small number of participants and aim to determine the drug's tolerability, dosage range, and potential side effects. If the results from these trials are promising, the drug may progress to later phases of clinical development.
Biomea Fusion, Inc. is the originator organization behind BMF-219. As the developer of the drug, they hold the intellectual property rights and are responsible for its research, development, and commercialization.
In summary, BMF-219 is a small molecule drug developed by Biomea Fusion, Inc. that targets menin. It is currently in Phase 1/2 of clinical development and has the potential to be used in the treatment of various diseases across multiple therapeutic areas. Further clinical trials will determine its safety and efficacy, and if successful, it may progress to later stages of development.