SOLA Biosciences presented validation information for SOL-257, Targeting at misfolded TDP-43 in ALS
SOLA Biosciences, a trailblazer in novel chaperone technology, is set to share its promising preliminary proof-of-concept data for SOL-257, a specific gene therapy aimed at misfolded TDP-43 in ALS. This presentation will be at the 22nd Annual Northeast ALS Consortium Meeting, scheduled in Florida on October 5.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TDP-43 is a critical nuclear protein with significant roles in the processing of DNA and RNA. In 97% of ALS instances, however, TDP-43 is mislocated to the cytoplasm. The detrimental effects of ALS are attributed to both the loss of nuclear TDP-43 function and the harmful consequences of cytoplasmic TDP-43 aggregates.
SOL-257, an AAV gene therapy, produces a therapeutic fusion protein. Acting together with HSP70, a crucial cellular chaperone, this uniquely structured fusion protein effectively presents TDP-43 in a misfolded state to HSP70, either facilitating the reformation of TDP-43 into its accurate conformation or driving its breakdown.
The in vivo proof-of-concept examination showed the beneficial impact of SOL-257 gene therapy in alleviating TDP-43 connected toxicity in the TDP-43-based and C9orf72-based ALS mouse models. Convincing results from behavioural tests strongly back the further preclinical development of SOL-257 as a potential treatment for ALS.
Dr. Akinori Hishiya, Chief Scientific Officer at SOLA, highlighted the potential of SOL-257 as a transformative therapy in ALS. He noted that efficacy in multiple genetic ALS mouse models shows that SOL-257's approach to misfolded TDP-43 treatment could potentially benefit virtually all ALS patients.
Dr. Gerry Cox, Chief Medical Officer at SOLA, expressed his excitement to finalise the preclinical studies necessary to bring SOL-257 to ALS clinical trials. Despite the current treatments available, the medical demand in ALS remains high, and the possibility of a wide-reaching one-time therapy brings renewed optimism to the ALS society.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
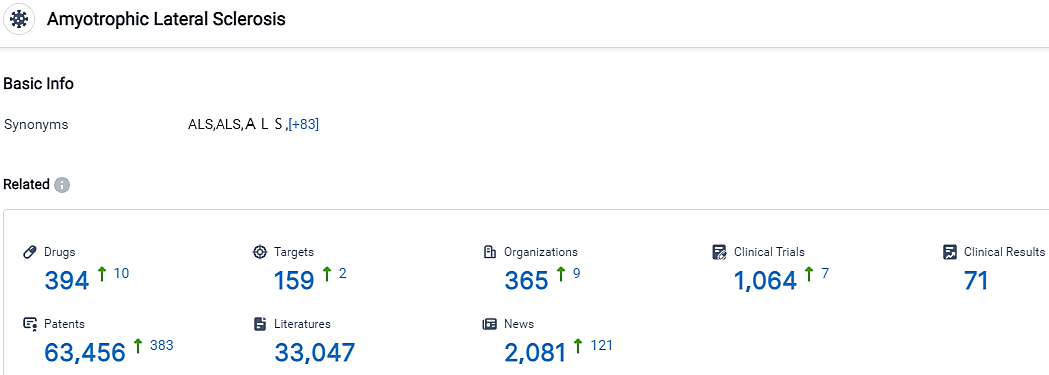
According to the data provided by the Synapse Database, As of October 11, 2023, there are 394 investigational drugs for the ALS, including 159 targets, 365 R&D institutions involved, with related clinical trials reaching 1064,and as many as 63456 patents.
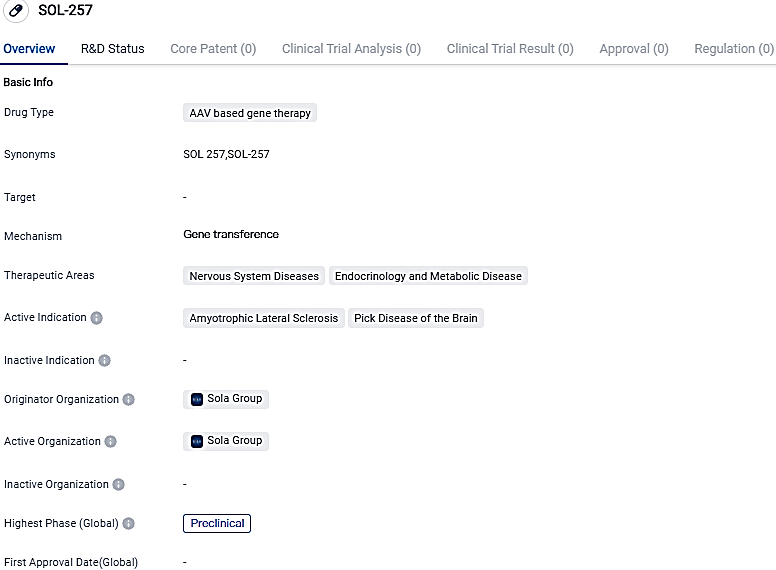
SOL-257 is currently in the preclinical phase and aims to address nervous system diseases and endocrinology and metabolic diseases, specifically targeting ALS and Pick Disease of the Brain. Further research and development are needed to determine the drug's safety and efficacy before it can progress to clinical trials.