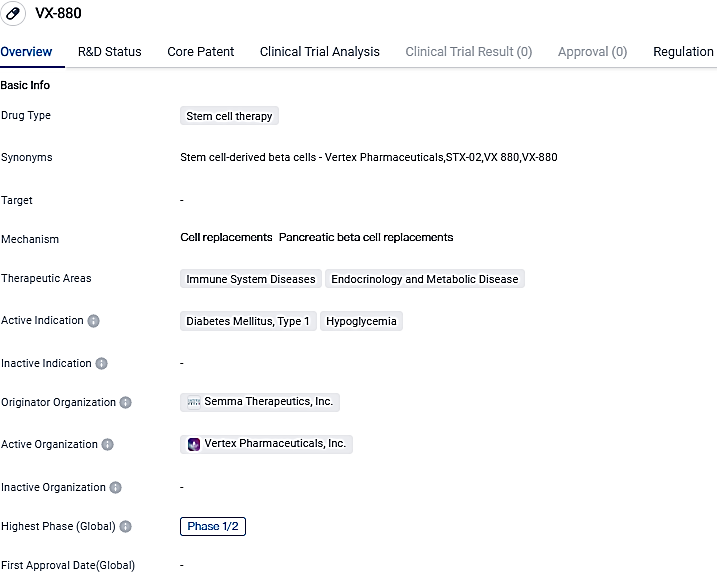
Vertex shares results of Phase 1/2 trial of VX-880 for Type 1 Diabetes
Vertex Pharmaceuticals Incorporated presented extensive data related to the patients treated in sections A and B of their Phase 1/2 clinical assessment trial of VX-880, an experimental solution derived from stem cells and is a completely differentiated islet cell therapy, it's aimed for individuals suffering from type 1 diabetes, that possess a deficient kind of hypoglycemic consciousness, and intense hypoglycemic episodes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Before undergoing VX-880 therapy, the six participants involved possessed long-term T1D without any self-produced insulin. They were on an average daily insulin dose of 34.0 units and had experienced several severe low-blood sugar episodes in the year leading up to screening.
In the more than three months of monitoring following treatment (Parts A and B), the patients have shown signs of islet cell injection and the ability to produce insulin in response to glucose based on Day 90 mixed-meal tolerance test results. All participants showed improved control of blood sugar levels across all parameters. This includes lower levels of HbA1c, enhanced time-in-range on continuous insulin observation, and either a decrease or complete stoppage in the usage of external insulin.
VX-880 therapy has been generally received well by all patients treated to this point. Most side effects were either minor or medium severity, with no severe AEs linked to the VX-880 treatment. As previously noted, a single participant experienced SHEs during the surgical period. No other SHEs have been observed in the study so far.
"We are amazed by the compelling evidence from the VX-880 project as seen by the improvements in all patients across all glycemic controls," stated Trevor Reichman, M.D., of the Department of Surgery at the University of Toronto. "This offers potential for an extremely promising experimental therapy with wide-reaching implications."
" The same VX-880 cells underpin our VX-264 cells-plus-device venture, and our hypoimmune islet cell project" commented Carmen Bozic, M.D., serving as Executive Vice President of Global Medicines Development and Medical Affairs and Chief Medical Officer at Vertex. "We are pursuing these potentially game-changing therapies with determination for the waiting patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
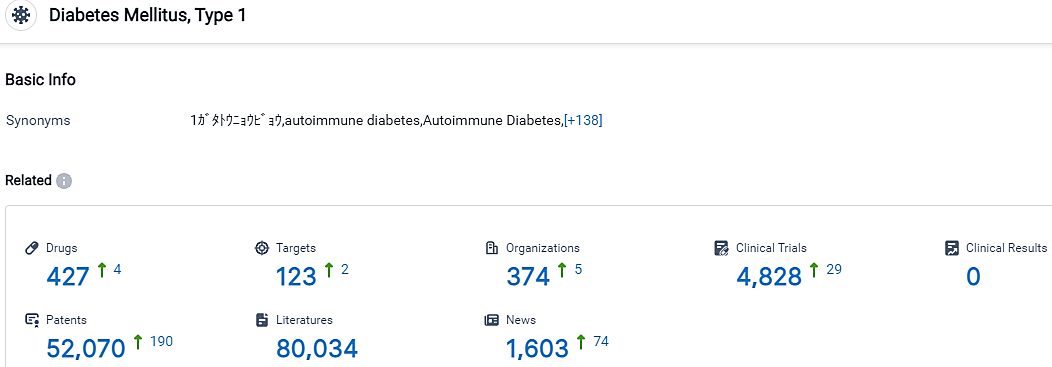
According to the data provided by the Synapse Database, As of October 11, 2023, there are 427 investigational drugs for the type 1 diabetes, including 123 targets, 374 R&D institutions involved, with related clinical trials reaching 4828,and as many as 52070 patents.
VX-880 was recently granted PRIME designation by the European Medicines Agency in March 2023, in addition to Fast Track Designation by the U.S. FDA in March 2021. PRIME designation is granted to innovative new therapies that have demonstrated the potential to significantly address an unmet medical need.