Analysis on the Research Progress of Dihydrofolate Reductase Inhibitor
DHFR, or dihydrofolate reductase, is an indispensable enzyme required for the survival of most prokaryotic and eukaryotic cells as it is involved in the biosynthesis of essential cellular components. DHFR has attracted a lot of attention as a molecular target for various diseases like cancer, bacterial infection, malaria, tuberculosis, dental caries, trypanosomiasis, leishmaniasis, fungal infection, influenza, Buruli ulcer, and respiratory illness. Various teams of researchers have reported different DHFR inhibitors to explore their therapeutic efficacy. Despite all the progress made, there is a strong need to find more novel leading structures, which may be used as better and safe DHFR inhibitors, especially against the microorganisms which are resistant to the developed drug candidates. A critical review of recent studies revealed that most novel DHFR inhibitor compounds either synthetically or naturally derived are characterized by the presence of heterocyclic moieties in their structure. Non-classical antifolates like trimethoprim, pyrimethamine, and proguanil are considered excellent templates to design novel DHFR inhibitors, and most of them have substituted 2,4-diamino pyrimidine motifs. Targeting DHFR has massive potential to be investigated for newer therapeutic possibilities to treat various diseases of clinical importance. Understanding the role of DHFR is essential for developing effective therapies and advancing pharmaceutical research.
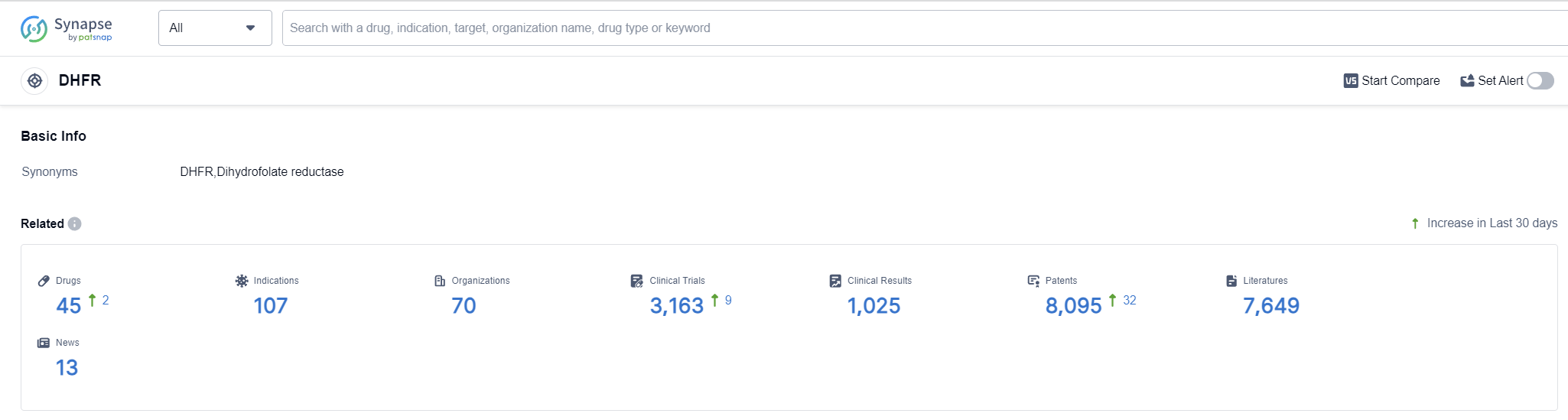
DHFR Competitive Landscape
According to Patsnap Synapse, as of 14 Oct 2023, there are a total of 45 DHFR drugs worldwide, from 70 organizations, covering 107 indications, and conducting 3163 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
In conclusion, the target DHFR in the pharmaceutical industry has seen significant development and progress. Pfizer Inc. is the leading company with the highest stage of development, having 3 approved drugs. The indications for the drugs under this target are diverse, ranging from Malaria to Rheumatoid Arthritis. Small molecule drugs are the most rapidly progressing drug type. China is the country with the highest number of approved drugs, indicating its growing presence in the pharmaceutical industry. The current competitive landscape suggests intense competition, especially in the small molecule drug category. The future development of the target DHFR is promising, with ongoing research and development efforts by various companies and countries/locations.
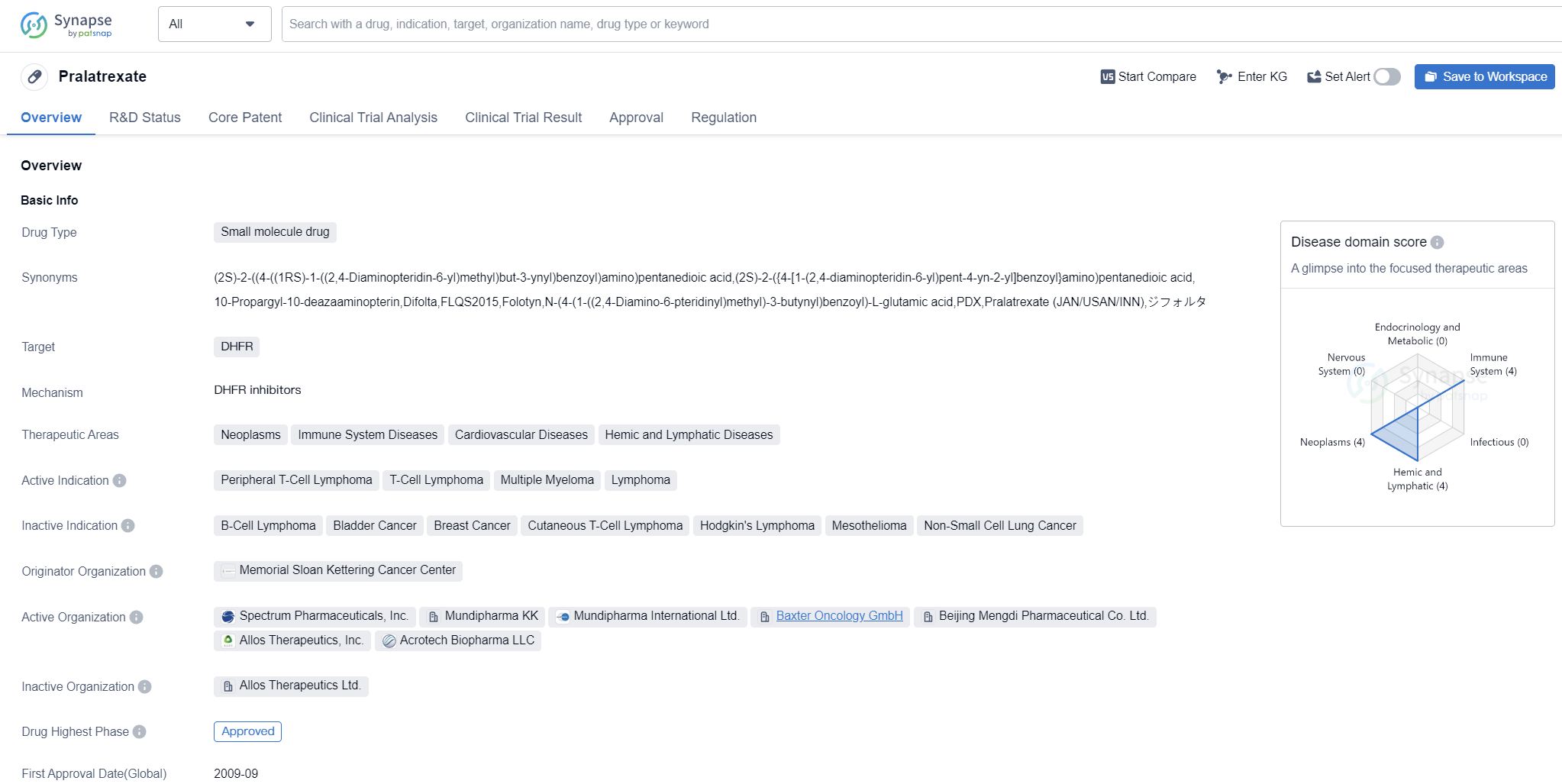
Key drug: pralatrexate
Pralatrexate is a small molecule drug that targets dihydrofolate reductase (DHFR). It is primarily used in the treatment of various diseases, including neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. The drug has been approved for the treatment of peripheral T-cell lymphoma, T-cell lymphoma, multiple myeloma, and lymphoma.
Pralatrexate was developed by the Memorial Sloan Kettering Cancer Center, a renowned organization in the field of cancer research and treatment. It has received approval for use in both the global market and China. The drug was first approved in the United States in September 2009.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of regulatory status, Pralatrexate has undergone priority review and accelerated approval processes. It has also been designated as an orphan drug, indicating its potential to treat rare diseases or conditions. These regulatory designations highlight the importance and urgency of the drug in addressing unmet medical needs.
The approval of Pralatrexate signifies its efficacy and safety in treating the indicated diseases. As a small molecule drug targeting DHFR, it is likely to have a specific mechanism of action that inhibits the activity of this enzyme. By doing so, it may disrupt the growth and proliferation of cancer cells, leading to improved patient outcomes.
The therapeutic areas in which Pralatrexate is indicated cover a wide range of diseases, including various types of cancers and immune system disorders. This suggests the drug's potential to address multiple medical conditions, making it a valuable asset in the pharmaceutical industry.
In summary, Pralatrexate is a small molecule drug developed by the Memorial Sloan Kettering Cancer Center. It has been approved for the treatment of peripheral T-cell lymphoma, T-cell lymphoma, multiple myeloma, and lymphoma. The drug targets DHFR and has shown efficacy in neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. Its regulatory status includes priority review, accelerated approval, and orphan drug designation. Pralatrexate's approval and therapeutic indications make it a significant drug in the field of biomedicine.
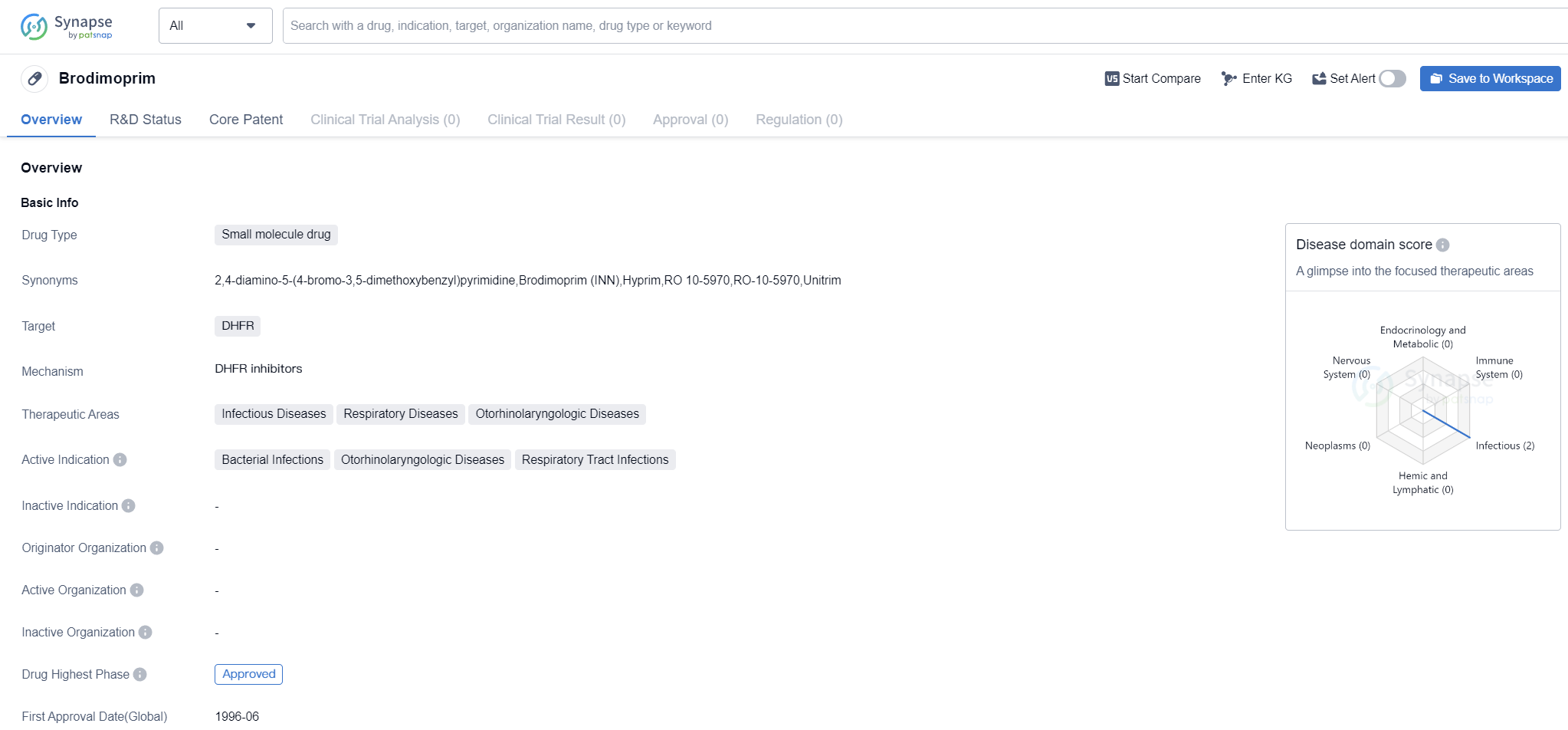
Brodimoprim
Brodimoprim is a small molecule drug that targets the enzyme dihydrofolate reductase (DHFR). It is primarily used in the treatment of infectious diseases, respiratory diseases, and otorhinolaryngologic diseases. The drug is indicated for bacterial infections, otorhinolaryngologic diseases, and respiratory tract infections.
Brodimoprim has reached the highest phase of development, which is approval, both globally and in China. The drug received its first approval in June 1996 in Spain. This indicates that it has been in the market for a considerable amount of time and has undergone rigorous testing and evaluation to ensure its safety and efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, brodimoprim is designed to interact with specific targets in the body, in this case, DHFR. By targeting this enzyme, the drug aims to inhibit its activity and disrupt the folate metabolism pathway in bacteria. This mechanism of action makes brodimoprim effective against bacterial infections, particularly those affecting the respiratory tract and otorhinolaryngologic system.
Infectious diseases pose a significant global health burden, and the availability of effective treatments is crucial in managing these conditions. Brodimoprim's approval in multiple countries, including Spain, suggests that it has demonstrated clinical benefits and met regulatory requirements for safety and efficacy.
Respiratory diseases, such as respiratory tract infections, are common and can range from mild to severe. Otorhinolaryngologic diseases, which affect the ears, nose, and throat, can also be a significant source of morbidity. Brodimoprim's indication for these therapeutic areas indicates its potential to address the unmet medical needs in these conditions.
Overall, brodimoprim is a small molecule drug that targets DHFR and is approved for the treatment of infectious diseases, respiratory diseases, and otorhinolaryngologic diseases. Its approval in Spain in 1996 and subsequent approval in China and other countries suggests its established efficacy and safety profile. The drug's mechanism of action and therapeutic indications make it a valuable option in managing bacterial infections, particularly those affecting the respiratory tract and otorhinolaryngologic system.