Decoding Naftifine Hydrochloride: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Naftifine Hydrochloride's R&D Progress
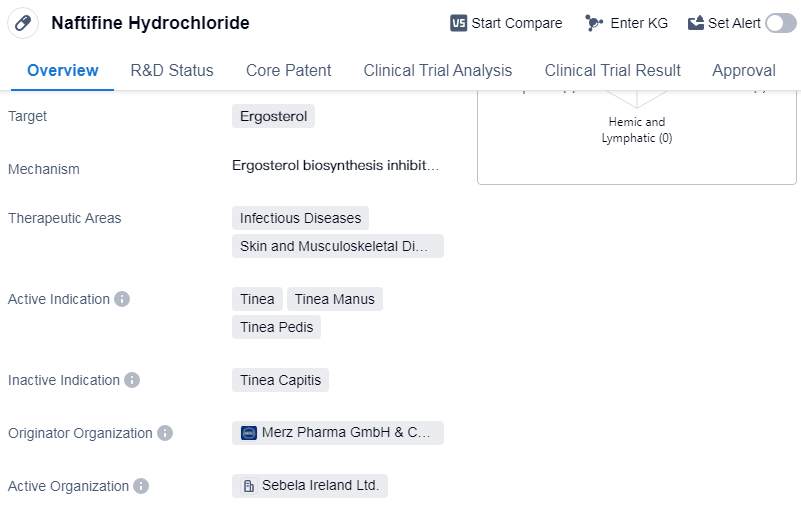
Naftifine Hydrochloride is a small molecule drug that targets ergosterol. It is primarily used in the treatment of infectious diseases, as well as skin and musculoskeletal diseases. The active indications for Naftifine Hydrochloride include tinea, tinea manus, and tinea pedis.
The drug was developed by Merz Pharma GmbH & Co. KGaA, a pharmaceutical company. It has received approval for use in multiple countries, including the United States. The first approval for Naftifine Hydrochloride was granted in February 1988 in the United States.
Naftifine Hydrochloride is classified as a small molecule drug, which means it is composed of low molecular weight compounds. Small molecule drugs are typically orally bioavailable and can easily penetrate cell membranes, allowing them to reach their target sites within the body.
The primary target of Naftifine Hydrochloride is ergosterol, which is a sterol found in the cell membranes of fungi, including those responsible for causing fungal infections. By targeting ergosterol, Naftifine Hydrochloride disrupts the integrity of the fungal cell membrane, leading to the death of the fungus.
The therapeutic areas in which Naftifine Hydrochloride is used includes infectious diseases and skin and musculoskeletal diseases, highlight its effectiveness in treating fungal infections. Naftifine Hydrochloride has undergone extensive clinical trials and has been proven to be safe and effective in treating these fungal infections. It has been approved in multiple countries, including the United States, demonstrates its widespread recognition and acceptance within the medical community.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Naftifine Hydrochloride: Ergosterol biosynthesis inhibitors
Ergosterol biosynthesis inhibitors are a class of compounds or drugs that specifically target and inhibit the biosynthesis of ergosterol in fungi. Ergosterol is a vital component of the fungal cell membrane, playing a similar role to cholesterol in animal cells. By inhibiting its biosynthesis, these inhibitors disrupt the integrity and function of the fungal cell membrane, leading to the inhibition of fungal growth and proliferation.
From a biomedical perspective, ergosterol biosynthesis inhibitors are of great importance in the field of antifungal therapy. Fungal infections, such as those caused by Candida or Aspergillus species, can be challenging to treat and often require specific antifungal agents. Ergosterol biosynthesis inhibitors offer a targeted approach to combat fungal infections by selectively inhibiting a crucial step in fungal cell membrane formation.
These inhibitors can be used in various forms, including topical creams, oral medications, or intravenous formulations, depending on the severity and location of the fungal infection. Common examples of ergosterol biosynthesis inhibitors include azoles (e.g., fluconazole, itraconazole) and allylamines (e.g., terbinafine).
It is worth noting that ergosterol biosynthesis inhibitors have minimal impact on mammalian cells because humans and other animals synthesize cholesterol instead of ergosterol. This selectivity makes them effective antifungal agents with relatively low toxicity to the host organism.
In summary, ergosterol biosynthesis inhibitors are compounds or drugs that specifically target the synthesis of ergosterol in fungi. They are used in biomedical applications to treat fungal infections by disrupting the integrity of the fungal cell membrane.
Drug Target R&D Trends for Naftifine Hydrochloride
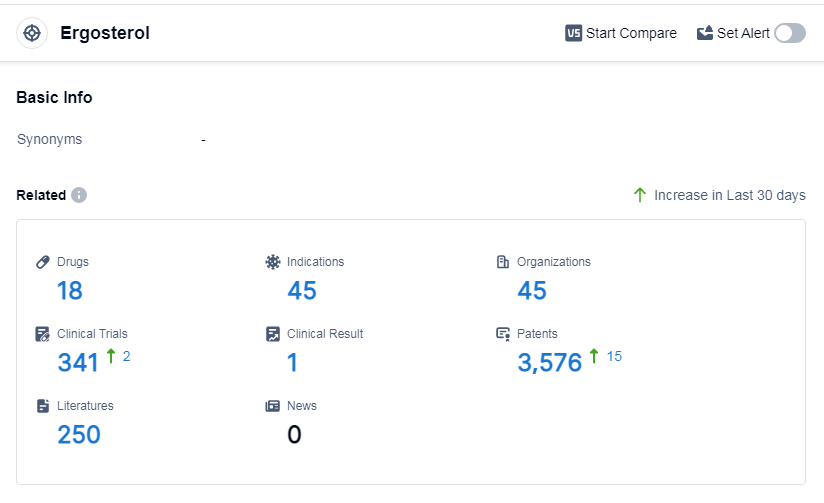
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 18 Ergosterol drugs worldwide, from 45 organizations, covering 45 indications, and conducting 341 clinical trials.
The analysis of the current competitive landscape and future development of target Ergosterol reveals that several companies are growing rapidly in this field, with drugs in the approved phase. These companies have made significant progress in R&D and have obtained regulatory approval for their drugs targeting Ergosterol. The indications for these drugs cover a wide range of fungal infections and related conditions, indicating the potential of drugs targeting Ergosterol in treating various fungal diseases. The dominance of small molecule drugs suggests intense competition, with the presence of biosimilars indicating the development of alternative versions of the innovative drugs. China, the United States, and Japan are leading in the development of drugs targeting Ergosterol, with other countries and regions also making progress. Overall, the target Ergosterol presents a promising opportunity for pharmaceutical companies, with potential for further advancements in the treatment of fungal infections.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Naftifine Hydrochloride is a small molecule drug developed by Merz Pharma GmbH & Co. KGaA. It targets ergosterol and is primarily used in the treatment of infectious diseases, skin, and musculoskeletal diseases. Its active indications include tinea, tinea manus, and tinea pedis. With its approval in multiple countries, Naftifine Hydrochloride has established itself as a reliable and effective treatment option for fungal infections.