ExploringMycophenolate Sodium's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Mycophenolate Sodium's R&D Progress
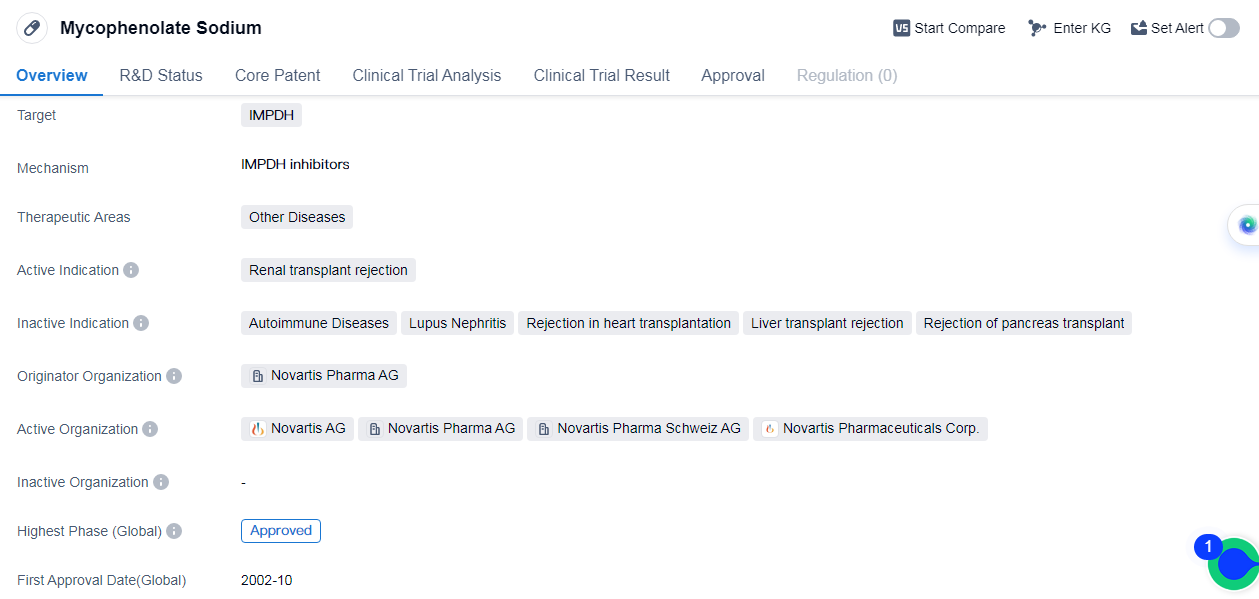
Mycophenolate Sodium is a small molecule drug that targets the enzyme ofinosine monophosphate dehydrogenase(IMPDH). It is primarily used in the treatment of renal transplant rejection. The drug was first approved by the regulatory authority in Switzerland in October 2002. The originator organization of Mycophenolate Sodium is Novartis Pharma AG.
Mycophenolate Sodium belongs to the therapeutic area of other diseases, indicating its potential use in treating various conditions beyond renal transplant rejection. The drug has reached the highest phase of development, with approvals in the global markets.
As a small molecule drug, Mycophenolate Sodium is designed to interact with specific molecular targets in the body, in this case, IMPDH. By targeting this enzyme, the drug aims to suppress the immune response and prevent rejection of transplanted kidneys.
The approval of Mycophenolate Sodium in Switzerland in 2002 marked an important milestone for the drug. It demonstrated its safety and efficacy in clinical trials, leading to its acceptance as a treatment option for renal transplant rejection. Subsequently, the drug received approval in other countries, including China.
Novartis Pharma AG, a renowned pharmaceutical company, is the originator organization behind Mycophenolate Sodium. Novartis has a strong presence in the global pharmaceutical market and is known for its expertise in developing innovative therapies.
The approval of Mycophenolate Sodium in the global markets highlights its potential as an effective treatment for renal transplant rejection. This approval signifies that the drug has undergone rigorous testing and evaluation, meeting the necessary regulatory standards.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Mycophenolate Sodium: IMPDH inhibitors
IMPDH inhibitors are a type of drug that targets and inhibits the enzyme of IMPDH. This enzyme plays a crucial role in the synthesis of guanine nucleotides, which are essential for DNA and RNA production. By inhibiting IMPDH, these inhibitors can disrupt the production of guanine nucleotides, leading to a decrease in DNA and RNA synthesis.
From a biomedical perspective, IMPDH inhibitors are primarily used in the treatment of certain diseases, particularly autoimmune diseasesand organ transplant rejection. These inhibitors are effective in suppressing the immune response by reducing the proliferation of lymphocytes, which are immune cells involved in the inflammatory process. By inhibiting IMPDH, these drugs can dampen the immune system's activity and prevent it from attacking healthy tissues or rejecting transplanted organs.
Some examples of IMPDH inhibitors include mycophenolate mofetil and mycophenolic acid, which are commonly used as immunosuppressive agents in transplantation medicine. These drugs are prescribed to prevent organ rejection in transplant recipients. Additionally, IMPDH inhibitors have also shown potential in the treatment of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and psoriasis.
It's important to note that the use of IMPDH inhibitors can have side effects, including gastrointestinal disturbances, bone marrow suppression, and increased susceptibility to infections. Therefore, these drugs are typically prescribed under medical supervision, and patients need to be closely monitored for any adverse reactions.
Drug Target R&D Trends for Mycophenolate Sodium
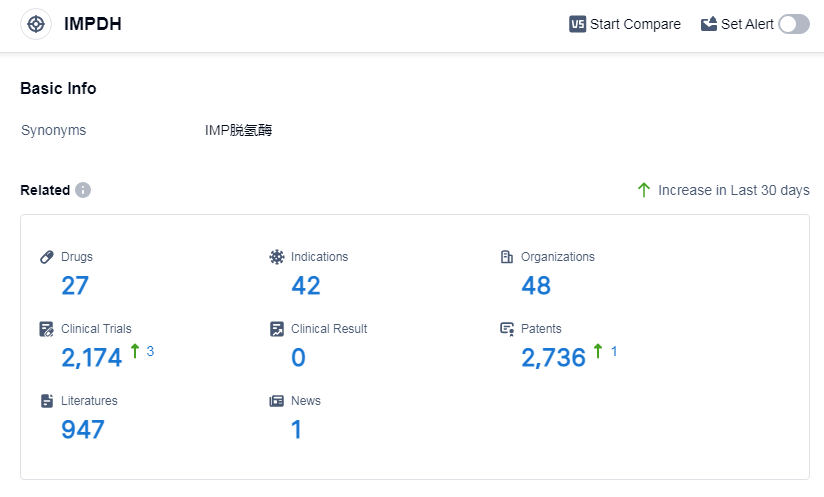
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 27 IMPDH drugs worldwide, from 48 organizations, covering 42 indications, and conducting 2174 clinical trials.
Based on the analysis of the data provided, the current competitive landscape of the target IMPDH is dominated by companies such as Roche Holding AG, Merck & Co., Inc., and Bausch Health Cos., Inc. These companies have the highest number of drugs in the approved phase, indicating their advanced stage of development. The most rapidly progressing drug types under the target IMPDH are small molecule drugs and interferons. The countries or locations developing fastest under the target IMPDH are the United States, European Union, China, and Japan. China has shown significant progress with 4 drugs approved under the target IMPDH.
Overall, the target IMPDH presents opportunities for further research and development, particularly in the areas of hepatitis C, renal transplant rejection, and lupus nephritis. The pharmaceutical industry should continue to focus on the development of small molecule drugs and explore the potential of interferons in treating diseases related to the target IMPDH. Additionally, collaborations and partnerships between companies in different countries can contribute to the future development of the target IMPDH.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Mycophenolate Sodium is a small molecule drug developed by Novartis Pharma AG. It targets the enzyme IMPDH and is primarily used in the treatment of renal transplant rejection. The drug has received approvals in Switzerland and China, indicating its efficacy and safety. Its approval in 2002 marked an important milestone in the field of biomedicine, providing a valuable treatment option for patients undergoing renal transplantation.