Analysis on the Research Progress of IL-6 inhibitors
Interleukin-6 (IL-6) is a 26-kDa secretory protein composed of 184 amino acids and two N-glycosylation sites, as well as four cysteine residues in the mature IL-6. IL-6 is a multifunctional cytokine that acts on multiple biological systems and organs. Bone marrow cells, such as macrophages and dendritic cells, produce IL-6 after Toll-like receptors (TLR) detect pathogens at sites of infection or tissue injury. IL-6 is essential for the proliferation of myeloma cells and the survival of plasma cells in the adaptive immune response because it induces the ability of plasma cells to differentiate and produce antibodies.
Initially defined as B-cell-stimulatory factor 2 (BSF-2), IL-6 promotes the synthesis of immunoglobulins through activated B cells, and its complementary deoxyribonucleic acid (DNA) was successfully cloned in 1986. It was later found that IL-6 is a prototype cytokine with pleiotropic biological effects on immune responses, acute-phase responses, and hematopoiesis. IL-6 is rapidly produced in response to infection or tissue injury and contributes to host defense.
On the other hand, overproduction of IL-6 is associated with the development of acute and life-threatening complications such as systemic inflammatory response syndrome (SIRS) and cytokine-release syndrome (CRS). IL-6 is a major cytokine in the cytokine storm, seen in some patients undergoing chimeric antigen receptor (CAR) T cell therapy.
IL-6 plays a key role in the pathogenesis of diseases such as rheumatoid arthritis, steatorrhea, inflammatory bowel disease, diabetes, vitiligo, psoriasis and multiple sclerosis.
IL-6 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 53 IL-6 drugs worldwide, from 119 organizations, covering 131 indications, and conducting 2004 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.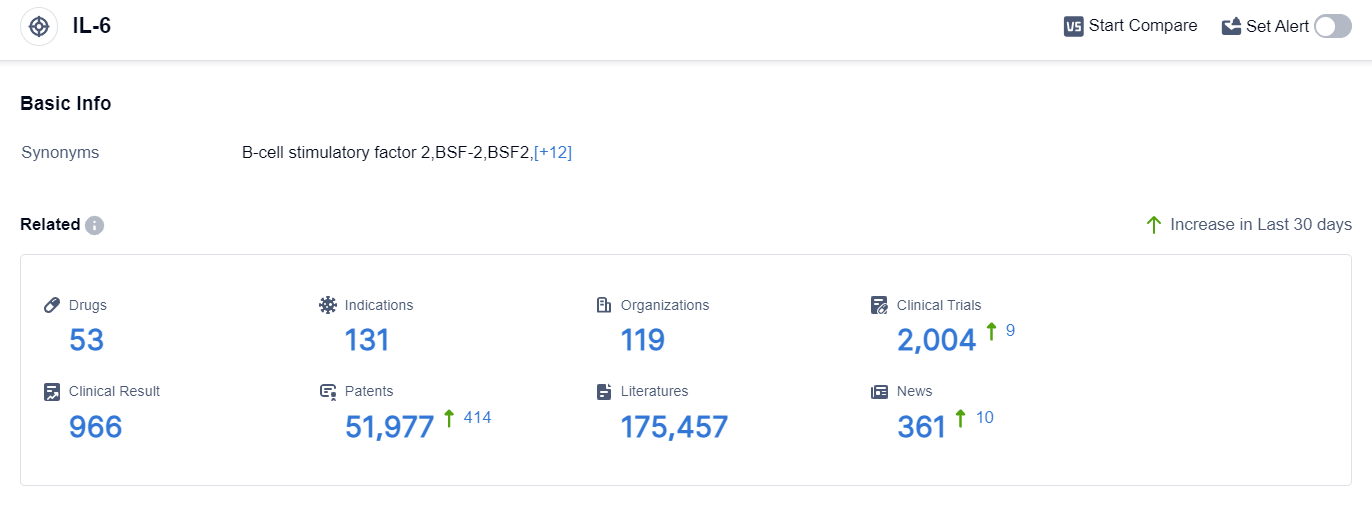
The analysis of the current competitive landscape and future development of target IL-6 reveals a strong focus on this target by various companies in the pharmaceutical industry. Bristol Myers Squibb Co., Roche Holding AG, and Johnson & Johnson are among the companies with the highest stage of development on this target.
Multiple drugs have been approved for indications such as rheumatoid arthritis, multiple myeloma, and COVID-19. Small molecule drugs, monoclonal antibodies, and molecular glues are progressing rapidly under the current targets.
The United States, China, and European Union are the leading countries/locations in the development of IL-6 targeted therapies. China has shown significant progress in this area, with multiple approved drugs. The analysis of the current competitive landscape and future development of target IL-6 indicates a promising future for the development of therapies targeting IL-6 and its associated indications.
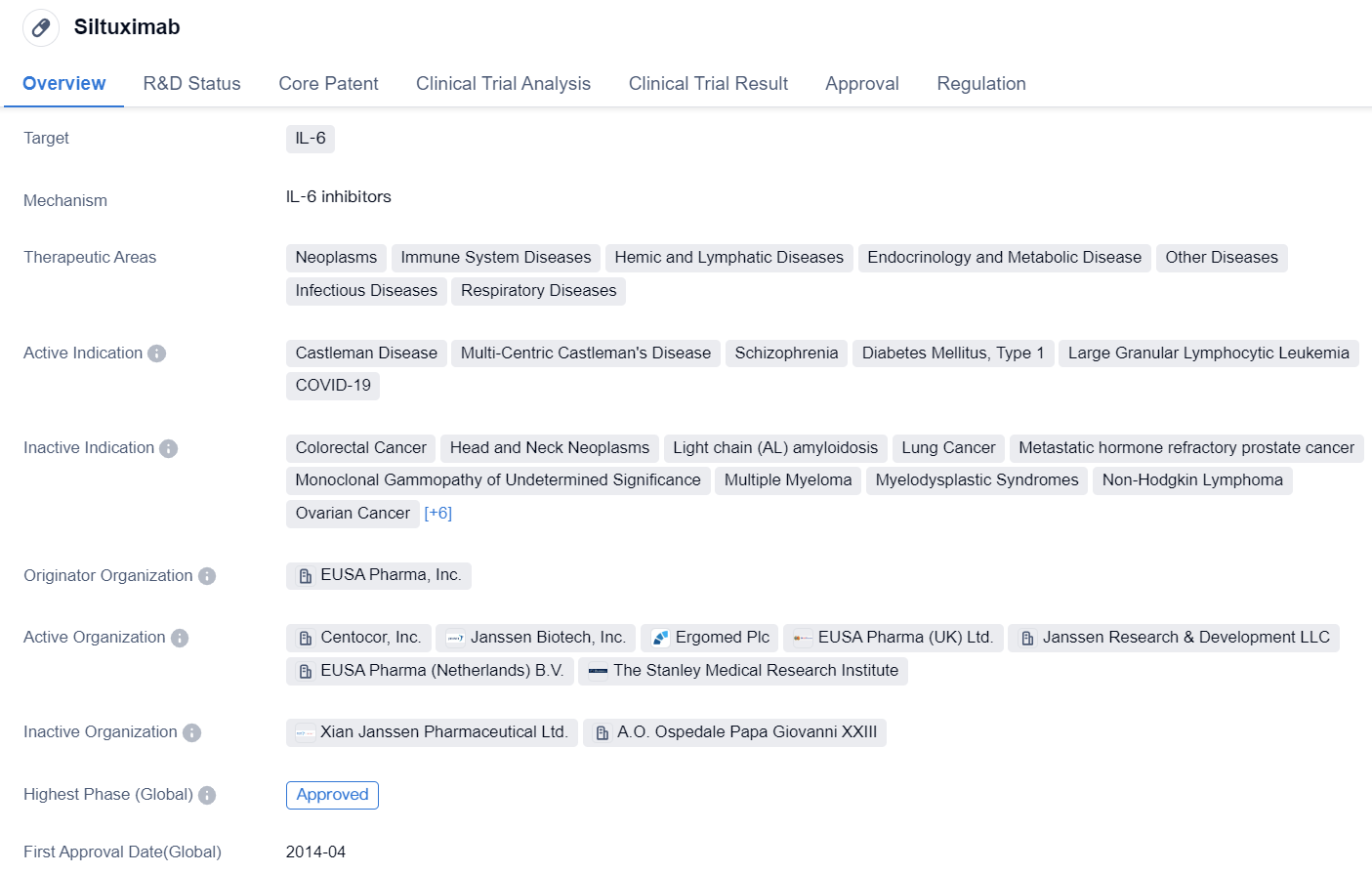
Approved for Market IL-6 Inhibitors: Siltuximab
Siltuximab is a monoclonal antibody drug that targets IL-6, a protein involved in various diseases. It has been approved for use in multiple therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, endocrinology and metabolic disease, other diseases, infectious diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating Castleman Disease, a rare disorder of the lymph nodes, as well as its variant, Multi-Centric Castleman's Disease. It has also been investigated for its potential in treating schizophrenia, diabetes mellitus type 1, large granular lymphocytic leukemia, and even COVID-19.
Siltuximab was developed by EUSA Pharma, Inc., and it received its first approval in the United States in April 2014. The drug has also been approved in China. Its approval in both countries indicates its potential global impact.
In terms of regulations, Siltuximab falls under the category of overseas new drugs urgently needed in clinical settings and has been designated as an orphan drug. This designation is given to drugs that treat rare diseases and provides certain incentives to the manufacturer, such as market exclusivity and financial benefits.
Overall, Siltuximab is a monoclonal antibody drug that targets IL-6 and has shown promise in various therapeutic areas. Its approval in the United States and China, as well as its designation as an orphan drug, highlight its potential in addressing unmet medical needs. Further research and clinical trials may reveal additional indications and expand its usage in the field of biomedicine.
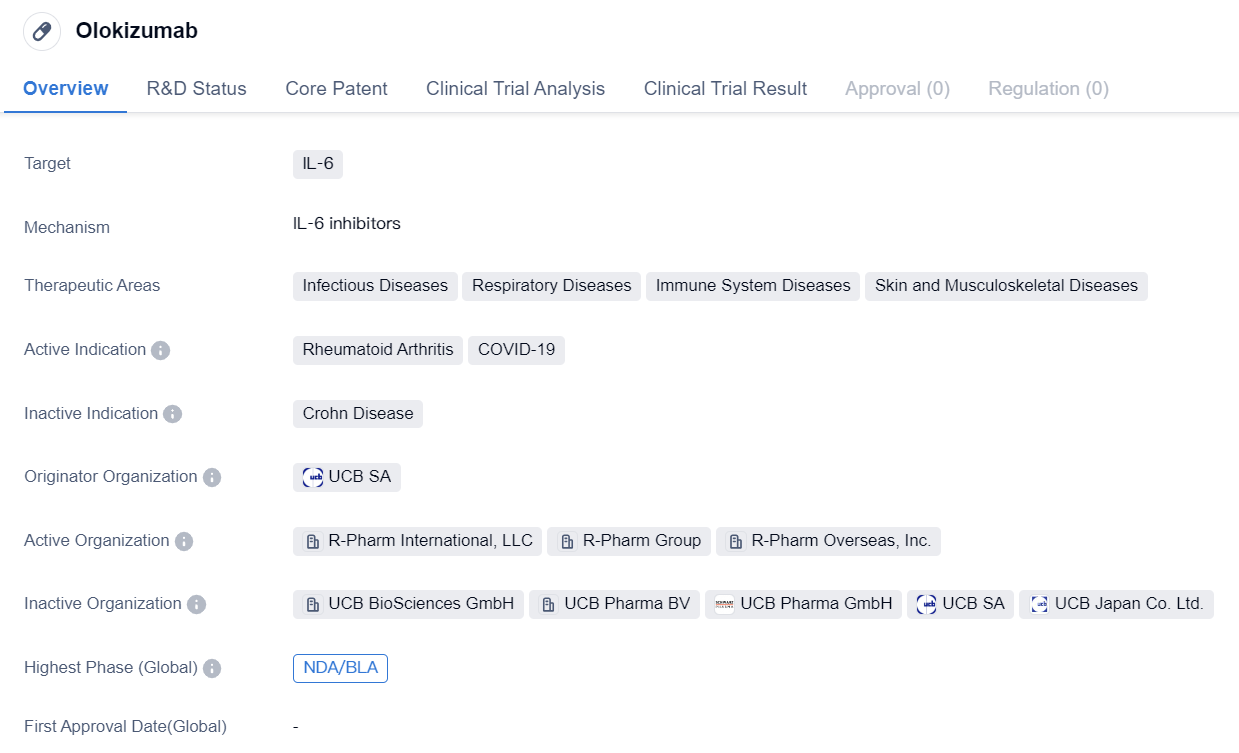
IL-6 inhibitors that have entered the NDA/BLA stage: Olokizumab
Olokizumab is a monoclonal antibody drug that targets IL-6, a protein involved in immune responses. It is being developed by UCB SA, a pharmaceutical company. The drug is primarily intended for the treatment of Rheumatoid Arthritis, a chronic inflammatory disorder that affects the joints. However, it is also being investigated for its potential use in COVID-19, the respiratory disease caused by the novel coronavirus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of therapeutic areas, Olokizumab is being explored for its efficacy in treating various infectious diseases, respiratory diseases, immune system diseases, as well as skin and musculoskeletal diseases. This suggests that the drug has a broad range of potential applications beyond its primary indication for Rheumatoid Arthritis.
In terms of its development stage, Olokizumab has reached the NDA/BLA stage globally. NDA stands for New Drug Application, which is the final step in the drug approval process in the United States. BLA stands for Biologics License Application, which is the equivalent process for biologic drugs. This indicates that the drug has completed clinical trials and is currently under review by regulatory authorities for potential approval.
In China, Olokizumab has reached Phase 3, which is the final stage of clinical trials before seeking regulatory approval. This suggests that the drug is progressing well in its development in China and may soon be available for patients in this market.
Overall, Olokizumab is a monoclonal antibody drug that targets IL-6 and is being developed by UCB SA. It has shown promise in the treatment of Rheumatoid Arthritis and is also being investigated for its potential use in COVID-19. With its broad therapeutic areas and advanced development stages, Olokizumab has the potential to address unmet medical needs in various disease areas and may provide new treatment options for patients.