Janssen applies to European Medicines Agency for approval to use RYBREVANT (amivantamab) plus chemotherapy for newly diagnosed advanced NSCLC patients with EGFR Exon 20 Insertion Mutations
The Janssen Pharmaceutical Companies of Johnson & Johnson have initiated a submission for a Type II extension of indication to the European Medical Agency. This application looks to gain acceptance for the usage of RYBREVANT® (amivantamab) combined with chemotherapy as a premier treatment strategy for adult patients suffering from advanced non-small cell lung cancer that harbors activating mutations in the epidermal growth factor receptor (EGFR) exon 20 insertion.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 Individuals diagnosed with advanced or metastatic NSCLC harboring activating EGFR exon 20 insertion mutations frequently confront a grim likelihood of survival, necessitating the urgent development of fresh treatment strategies from the initial therapy phase," stated Martin Vogel, Area Leader for Oncology Therapeutics in EMEA at Janssen-Cilag GmbH. "Our proposal to EMA demonstrates our firm dedication to modifying the progress of lung cancer by introducing early and targeted treatment alternatives for suitable patients."
Individuals diagnosed with advanced or metastatic NSCLC harboring activating EGFR exon 20 insertion mutations frequently confront a grim likelihood of survival, necessitating the urgent development of fresh treatment strategies from the initial therapy phase," stated Martin Vogel, Area Leader for Oncology Therapeutics in EMEA at Janssen-Cilag GmbH. "Our proposal to EMA demonstrates our firm dedication to modifying the progress of lung cancer by introducing early and targeted treatment alternatives for suitable patients."
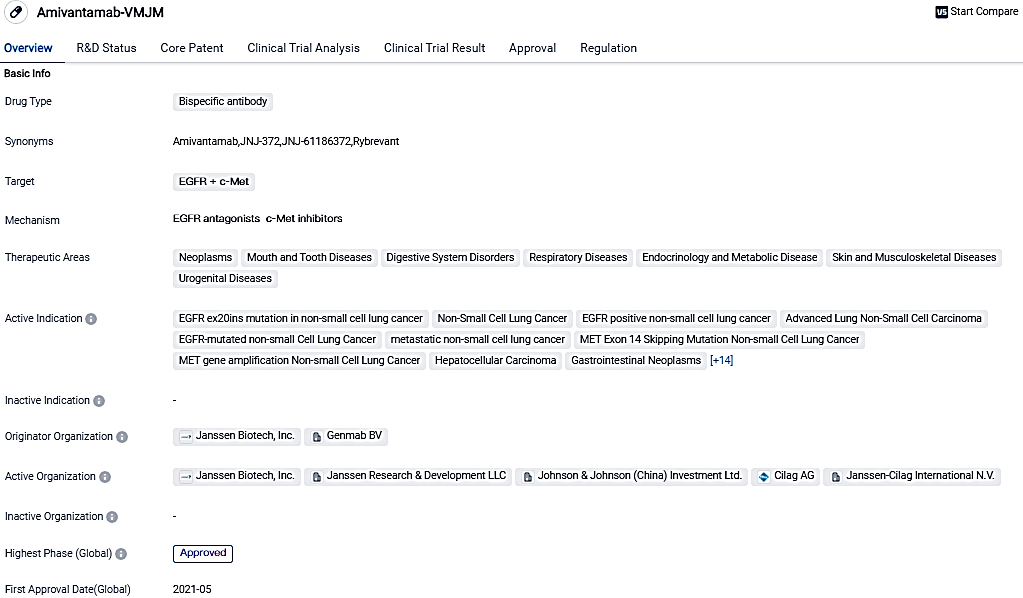
In December 2021, Amivantamab received conditional marketing approval from the European Commission, becoming the inaugural completely human, bispecific antibody designated for the solo treatment of patients with NSCLC carrying EGFR exon 20 insertion mutations, subsequent to unsuccessful platinum-centric therapy.
The most recent proposal to the EMA is backed by data procured from the PAPILLON Phase 3 clinical trial – a randomized, non-blinded study examining the effectiveness and safety of amivantamab used in combination with chemotherapy as a leading therapy for patients diagnosed with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
"PAPILLON represents the premier randomized Phase 3 trial to have its results released for NSCLC patients harboring EGFR exon 20 insertion mutations," voiced Kiran Patel, M.D., Vice President, Clinical Development for Solid Tumors at Janssen Research & Development, LLC. "We anticipate collaborating with the EMA to introduce this prospective new indication to the lung cancer population as promptly as feasible."
The proposal sent to the EMA trails the recent submission of a supplemental biologics license application to the U.S. FDA in pursuit of expanded approval for Amivantamab as a pioneering combination treatment for advanced or metastatic patients with NSCLC demonstrating EGFR exon 20 insertion mutations.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
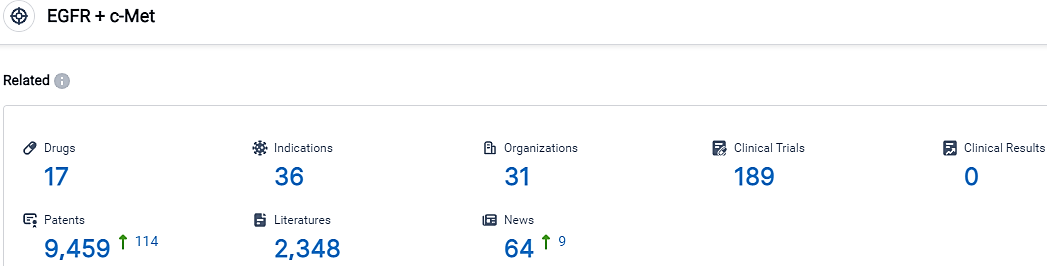
According to the data provided by the Synapse Database, As of October 17, 2023, there are 17 investigational drugs for the EGFR and c-Met target, including 36 indications, 31 R&D institutions involved, with related clinical trials reaching 189,and as many as 9459 patents.
Amivantamab, a full-human EGFR-MET bispecific antibody, directs immune cells to target tumours exhibiting activation and resistance of EGFR and MET mutations and amplifications. In December 2021, the European Commission provisionally authorized the use of amivantamab for treating adult patients suffering from advanced NSCLC.