Analysis on the Research Progress of Protease inhibitors
Proteases are enzymes that play a crucial role in the human body by breaking down proteins into smaller peptides or amino acids. These enzymes are involved in various physiological processes, including digestion, blood clotting, immune response, and cell signaling. Proteases help in the breakdown of dietary proteins into absorbable nutrients, aiding in digestion and nutrient absorption. They also regulate blood clotting by activating clotting factors. Additionally, proteases are involved in immune response by degrading foreign proteins and pathogens. Furthermore, these enzymes participate in cell signaling pathways, influencing various cellular processes such as growth, differentiation, and apoptosis. Overall, proteases are essential for maintaining proper protein homeostasis and ensuring the normal functioning of the human body.
Protease Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 48 Protease drugs worldwide, from 60 organizations, covering 66 indications, and conducting 228 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.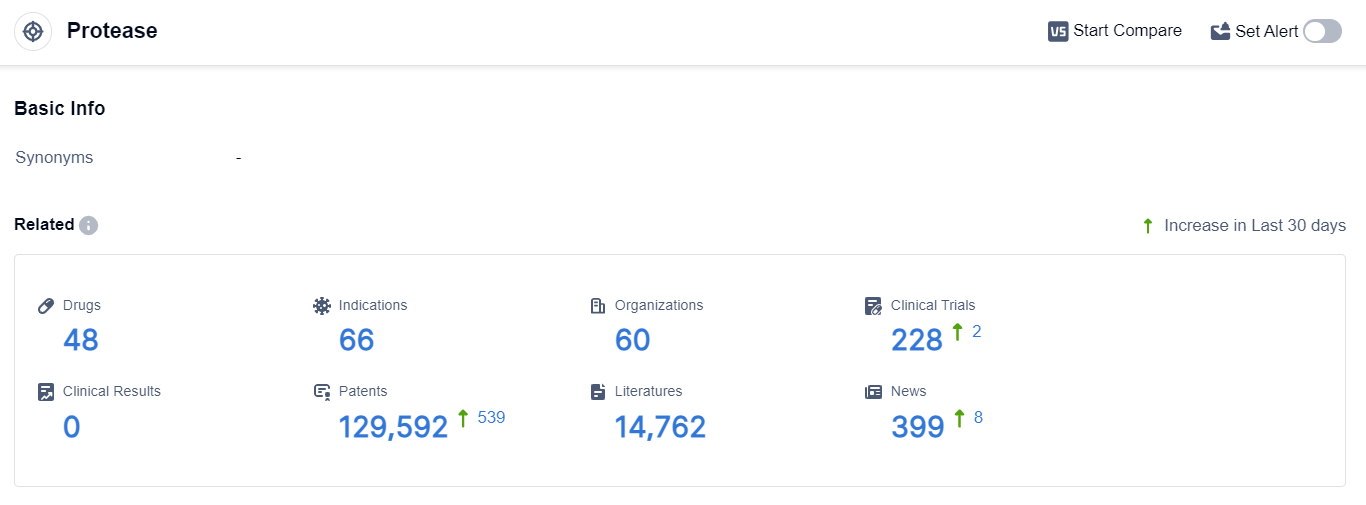
The analysis of the current competitive landscape and future development of target Protease reveals several key findings. Shanghai Pharmaceuticals Holding Co., Ltd., Propanc Biopharma, Inc., and Onconova Therapeutics, Inc. are among the companies growing fastest under this target.
The highest stage of development is the "Approved" phase, with drugs targeting various indications. Enzyme, Small molecule drug, and Chemical drugs are the drug types progressing most rapidly, indicating intense competition.
China, along with other countries such as the European Union, United States, and United Kingdom, is developing rapidly in the field of Protease inhibitors. The future development of target Protease holds promise for the treatment of various medical conditions and the advancement of the pharmaceutical industry.
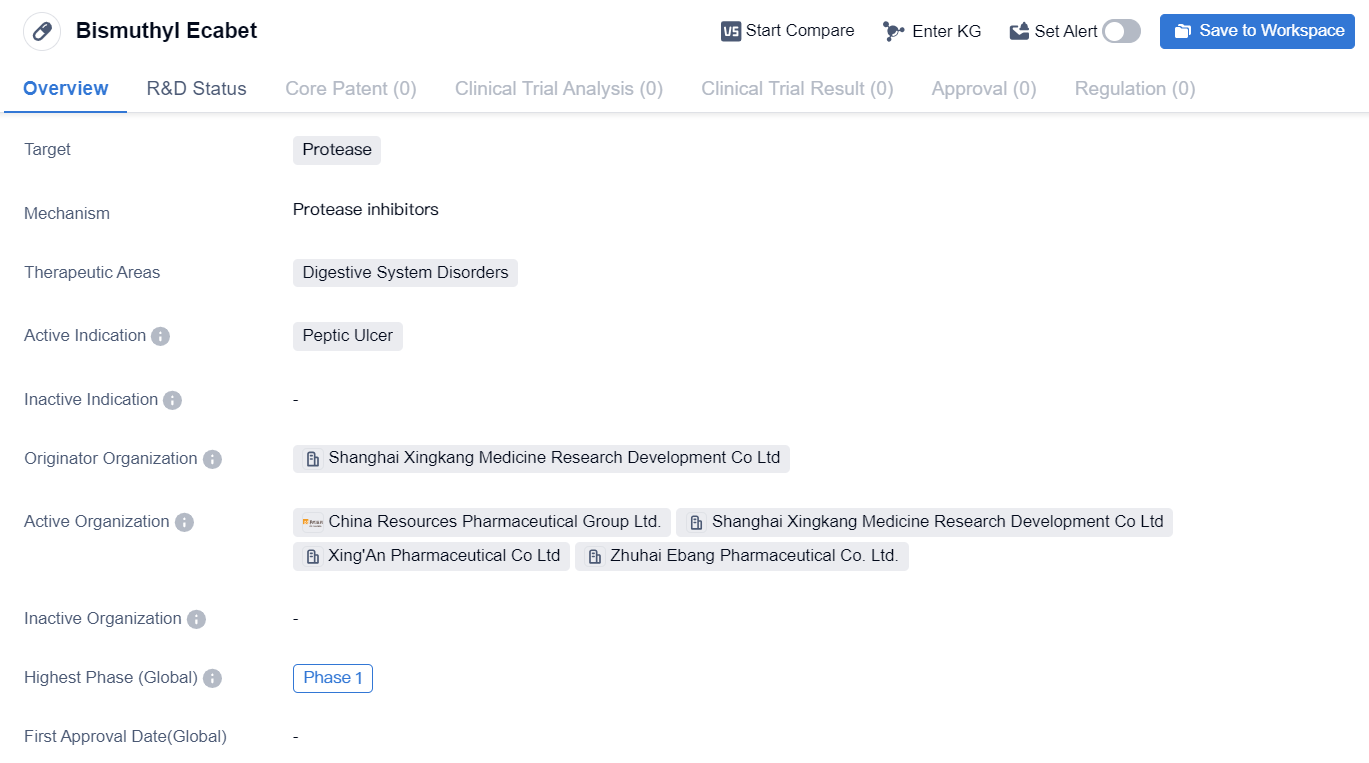
Protease inhibitors entering Phase I clinical trials: Bismuthyl Ecabet
Bismuthyl Ecabet is a small molecule drug that falls under the therapeutic area of digestive system disorders. It specifically targets protease enzymes and is primarily indicated for the treatment of peptic ulcers. The drug is being developed by Shanghai Xingkang Medicine Research Development Co Ltd, an originator organization based in China.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As per the available information, Bismuthyl Ecabet has reached Phase 1 of clinical development, both globally and in China. Phase 1 trials are typically conducted to evaluate the safety and tolerability of a drug in a small group of healthy volunteers or patients. These trials aim to determine the appropriate dosage range and identify any potential side effects.
The drug's mechanism of action, efficacy, and specific details regarding its development progress beyond Phase 1 are not provided in the given information. Therefore, it is difficult to assess the drug's potential impact or its competitive position in the market.
However, Bismuthyl Ecabet's focus on targeting protease enzymes suggests that it may have the potential to modulate the digestive system's physiological processes. Proteases play a crucial role in the breakdown of proteins, and their dysregulation can contribute to various digestive disorders, including peptic ulcers. By targeting proteases, Bismuthyl Ecabet may aim to restore the balance and alleviate the symptoms associated with peptic ulcers.
It is worth noting that Phase 1 is an early stage of clinical development, and many drugs do not progress beyond this phase due to safety or efficacy concerns. Therefore, further research and clinical trials will be necessary to determine the drug's potential effectiveness and safety profile.
In conclusion, Bismuthyl Ecabet is a small molecule drug being developed by Shanghai Xingkang Medicine Research Development Co Ltd for the treatment of peptic ulcers. While the drug's specific details, mechanism of action, and efficacy are not provided, its focus on targeting protease enzymes suggests potential therapeutic benefits. However, as the drug is currently in Phase 1 of clinical development, further research and trials will be required to assess its potential impact in the field of digestive system disorders.
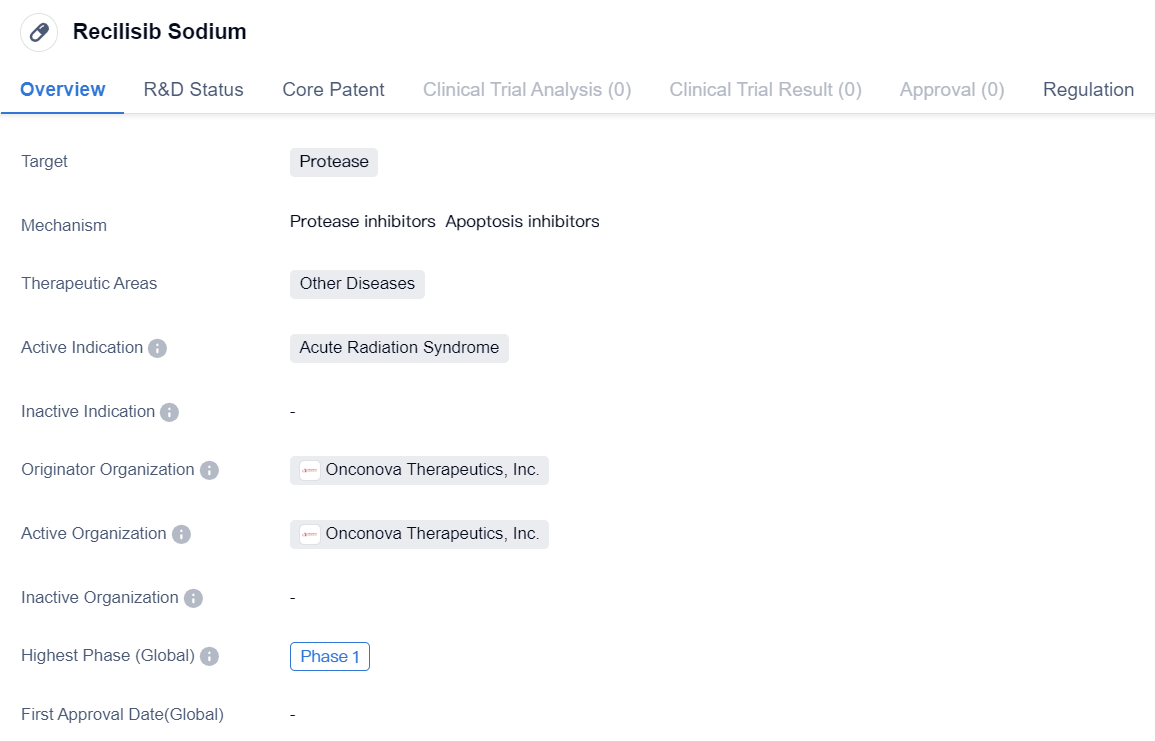
Protease inhibitors entering Phase I clinical trials: Recilisib Sodium
Recilisib Sodium is a small molecule drug that targets protease and is being developed for the treatment of acute radiation syndrome (ARS). It is being developed by Onconova Therapeutics, Inc., a pharmaceutical company specializing in the development of novel therapies for cancer and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ARS is a condition that occurs when the body is exposed to high levels of radiation, such as during a nuclear accident or radiation therapy for cancer. It can cause severe damage to the bone marrow, gastrointestinal tract, and other vital organs. Currently, there are limited treatment options available for ARS, making the development of new therapies crucial.
Recilisib Sodium is currently in Phase 1 of clinical development, which means it is being tested in a small group of healthy volunteers to evaluate its safety, dosage, and potential side effects. Phase 1 trials are the first step in the clinical development process and are designed to gather preliminary data on the drug's safety and pharmacokinetics.
The drug has been granted orphan drug status, which provides incentives and support for the development of drugs that treat rare diseases or conditions. Orphan drug designation is granted by regulatory authorities to encourage the development of therapies for conditions that affect a small number of patients.
Based on the available information, Recilisib Sodium shows promise as a potential treatment for ARS. However, it is important to note that the drug is still in the early stages of development, and further clinical trials will be needed to determine its efficacy and safety profile. The pharmaceutical industry is constantly evolving, and the success of a drug in clinical trials can never be guaranteed.
In conclusion, Recilisib Sodium is a small molecule drug being developed by Onconova Therapeutics, Inc. for the treatment of acute radiation syndrome. It is currently in Phase 1 of clinical development and has been granted orphan drug status. Further research and clinical trials will be necessary to determine its potential as a treatment for ARS.