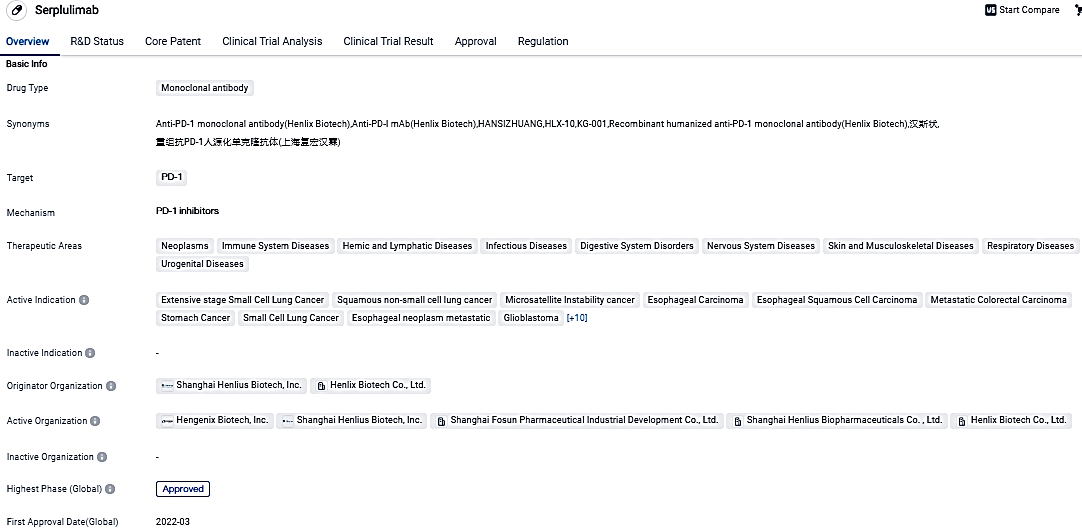
Henlius' Innovative Anti-PD-1 mAb HANSIZHUANG (Serplulimab) Receives Approval for Use in Treating ESCC
Shanghai Henlius Biotech, Inc. has made a public announcement that the NMPA has given approval to the NDA for an innovative fresh indication of HANSIZHUANG (serplulimab injection), an anti-PD-1 monoclonal antibody developed in-house, combined with drugs containing fluorouracil and platinum. This approval enables it for first-line usage in treating patients with PD-L1 positive, locally advanced/acurrent unresectable or metastatic esophageal squamous cell carcinoma (ESCC), thus offering a new treatment avenue for ESCC patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Until now, HANSIZHUANG has been officially utilized in the medical treatment of specific types of cancers, including microsatellite instability-high solid tumors, squamous non-small cell lung cancer, and extensive stage small cell lung cancer. In an effort to further aid patients, Henlius continually hones and improves the product range and strategic plan for HANSIZHUANG within the areas of lung cancer, gastrointestinal cancer, and various other forms of cancer.
Wenjie Zhang, who holds the Chairman and Executive Director positions at Henlius, commented, "The approval of HANSIZHUANG for ESCC is its fourth sanctioned application, indicating that this high-grade anti-PD-1 mAb offers a fresh glimmer of hope when dealing with the complex treatment of gastrointestinal cancer.
Since being introduced in March 2022, HANSIZHUANG has aided a broad spectrum of patients diagnosed with lung cancer, gastrointestinal cancer, and other types of tumors, thereby strengthening its positive image and robust market presence. Looking ahead, we intend to continue to utilize the power of HANSIZHUANG and unearth its full potential to expedite our market outreach and deliver cost-effective, ground-breaking treatment options to more global patients."
Expressing his views, Jason Zhu, Henlius's Executive Director, CEO, President, and CFO stated, "Henlius is highly motivated by unfulfilled clinical requirements and is fully committed to precision immunotherapy for tumor conditions. Our attention is on the key types of cancers, methodically widening the clinical application of HANSIZHUANG across numerous cancer kinds, while actively commencing immunotherapy combination trial studies globally. We eagerly await more favorable research outcomes pertaining to HANSIZHUANG to further bolster our contribution to cancer treatment for an increasing number of cancer patients.
From the Cancer Hospital of the Chinese Academy of Medical Sciences, Prof. Jing Huang, the chief principal investigator of ASTRUM-007 expressed, "ESCC represents the most frequently occurring pathology of esophageal cancer with a high level of clinical necessity and comparatively bleak prognosis rates overall. We have high expectations that serplulimab will be beneficial to a greater number of patients in clinical settings."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
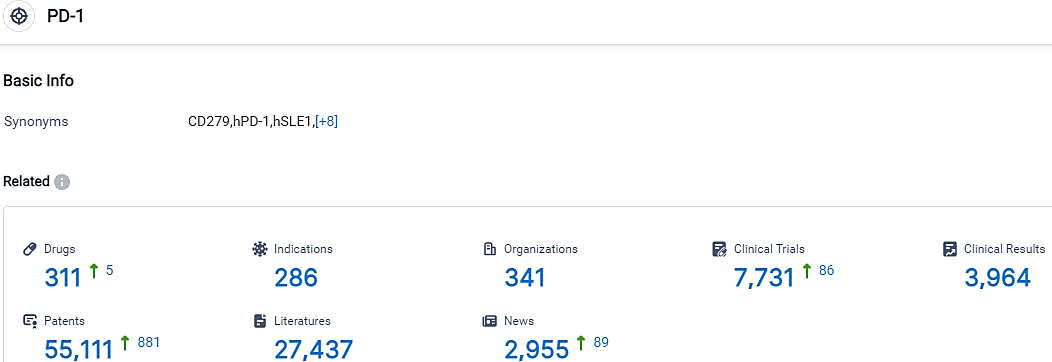
According to the data provided by the Synapse Database, As of September 28, 2023, there are 311 investigational drugs for the PD-1 target, including 286 indications,341 R&D institutions involved, with related clinical trials reaching 7731,and as many as 55111 patents.
The green light for serplulimab's use in China is a notable achievement for the medication and underlines the increasing importance of China's drug market. Given its wide range of potential treatments and regulatory backing, serplulimab may notably influence the biomedicine sector and enhance disease outcomes for various patient populations. Continued international endorsements and additional research could offer further data regarding the medication's effectiveness and risk assessment.