New Epigenetic Drug Targets: PRMT5 Inhibitors
Protein arginine methyltransferases (PRMTs) are catalytic enzymes for arginine methylation, capable of methylating a variety of proteins and playing pivotal roles in biological processes such as gene expression, splicing, and DNA damage repair.
Currently, 9 members of the PRMTs family has been identified, divided into three major types. Type I, catalyzes the formation of asymmetric dimethylarginine and includes PRMT1, PRMT2, PRMT3, PRMT4, PRMT6, PRMT8. Type II catalyzes the formation of symmetric dimethylarginine and includes PRMT5 and PRMT9. Type III is responsible for the formation of monomethylarginine and includes only PRMT7.
PRMT5 is the most highly studied subtype of PRMT, forming a heterooctameric complex with Methylosome protein 50 (MEP50), enabling the symmetrical dimethylation of a variety of substrates, including histone and non-histone proteins. PRMT5 histone substrates primarily include H4 residue Arg3 (H4R3), H3 residue Arg2 (H3R2), Arg8 (H3R8), and H2A residue Arg3 (H2AR3), whose symmetric dimethylation is related to gene transcription regulation. Non-histone substrates of PRMT5 include Sm proteins, nucleolar proteins, RAF proteins, EGFR, androgen receptor (AR), tumor suppressor p53, IL-2, HOXA9, NFkB, KLF4, GATA4, PDCD4, E2F-1, SPT5, GM130, POLR2A, SREBP1a, Rad9, and more that are involved in regulating cellular signalling, differentiation, DNA repair, and mRNA splicing among other cellular processes.
Research has found that PRMT5 is overexpressed in various types of cancer, such as glioblastoma, melanoma, leukemia/lymphoma, multiple myeloma, prostate cancer, bladder urothelial carcinoma, ovarian cancer, lung cancer, gastric cancer, and colorectal cancer. Furthermore, PRMT5 expression is associated with a poor prognosis in tumor progression and metastasis.
Further research has shown that PRMT5 directly affects the growth and differentiation of tumor cells by regulating multiple genes such as p53, IL-2, TRAIL receptor, MITF, transcription factor E2F-1, and tumor suppressor gene 7. Additionally, PRMT5 has been found to constitute "synthetic lethal" partnerships with gene mutations like methylthioadenosine phosphorylase (MTAP), metabolic enzyme methionine adenosyltransferase 2 alpha (MAT2A), and others.
Both in vitro and in vivo studies have shown that inhibition or knockdown of PRMT5 significantly reduces cell proliferation, migration, and colony formation, but promotes apoptosis and cell cycle arrest. Furthermore, oral PRMT5 inhibitors demonstrate significant antitumor activity in lung tumor xenograft models. Thus, PRMT5 has become a new target for the development of epigenetic anti-cancer drugs.
PRMT5 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 41 PRMT5 drugs worldwide, from 39 organizations, covering 28 indications, and conducting 20 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.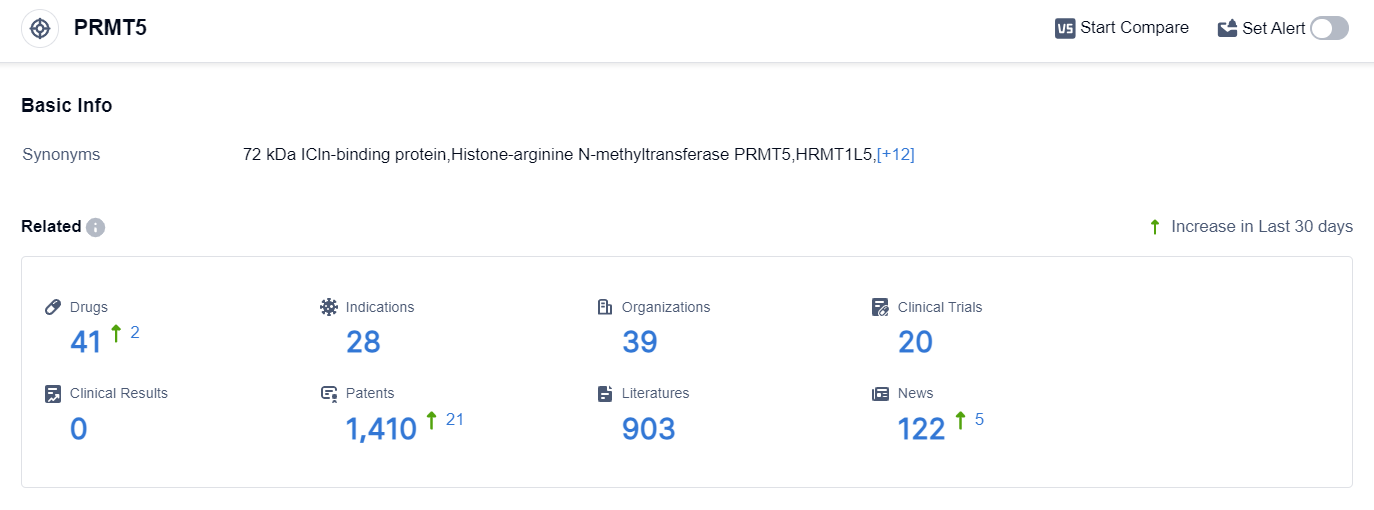
Based on the analysis of target PRMT5, it can be concluded that several companies, including GSK Plc, Ipsen SA, Tango Therapeutics, Inc., Amgen, Inc., Mirati Therapeutics, Inc., and Prelude Therapeutics, Inc., are actively involved in the research and development of drugs targeting PRMT5. These companies have drugs in various stages of development, with some reaching advanced stages such as Phase 2.
The indications for these drugs cover a wide range of cancer types, indicating the potential of PRMT5 as a therapeutic target. Small molecule drugs and chemical drugs are the most rapidly progressing drug types under the target PRMT5, with small molecule drugs being the primary focus.
The United States, China, and Canada are the countries/locations with the highest development activity, with the United States leading in Preclinical stage and China showing progress in Phase 1. Overall, the current competitive landscape suggests a promising future for the development of PRMT5 inhibitors in the pharmaceutical industry.
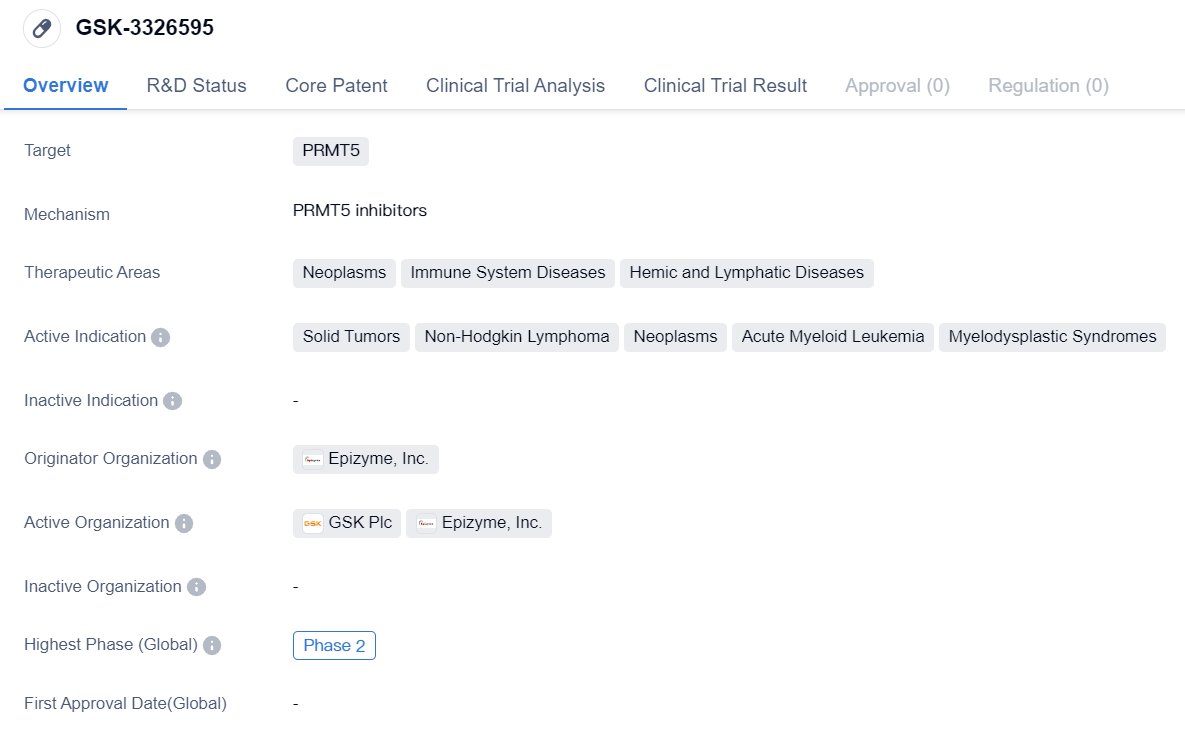
PRMT5 inhibitors entering Phase II clinical studies: GSK-3326595
GSK-3326595 is a small molecule drug that is being developed by Epizyme, Inc. It is designed to target PRMT5, a protein involved in various cellular processes. The drug is being investigated for its potential therapeutic applications in neoplasms, immune system diseases, and hemic and lymphatic diseases.
The active indications for GSK-3326595 include solid tumors, non-Hodgkin lymphoma, neoplasms, acute myeloid leukemia, and myelodysplastic syndromes. These are all serious and potentially life-threatening conditions that affect different parts of the body, including the blood, lymph nodes, and various organs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, GSK-3326595 is in Phase 2 of clinical development, which means that it has already undergone initial testing in humans and has shown promising results in terms of safety and efficacy. Phase 2 trials typically involve a larger number of participants and aim to further evaluate the drug's effectiveness and side effects.
As a small molecule drug, GSK-3326595 has the advantage of being able to penetrate cells and interact with specific targets, such as PRMT5. This targeted approach can potentially lead to more effective treatment options with fewer side effects compared to traditional chemotherapy or radiation therapy.
Epizyme, Inc. is the originator organization behind GSK-3326595. They are a biopharmaceutical company focused on developing novel therapies for patients with genetically defined cancers and other diseases. With their expertise in epigenetics and targeted therapies, Epizyme is dedicated to advancing the field of biomedicine and improving patient outcomes.
In summary, GSK-3326595 is a small molecule drug being developed by Epizyme, Inc. It targets PRMT5 and has potential applications in neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug is currently in Phase 2 of clinical development and shows promise in treating solid tumors, non-Hodgkin lymphoma, acute myeloid leukemia, and myelodysplastic syndromes. Epizyme, Inc. is the organization behind the development of this innovative drug, aiming to provide new treatment options for patients in need.
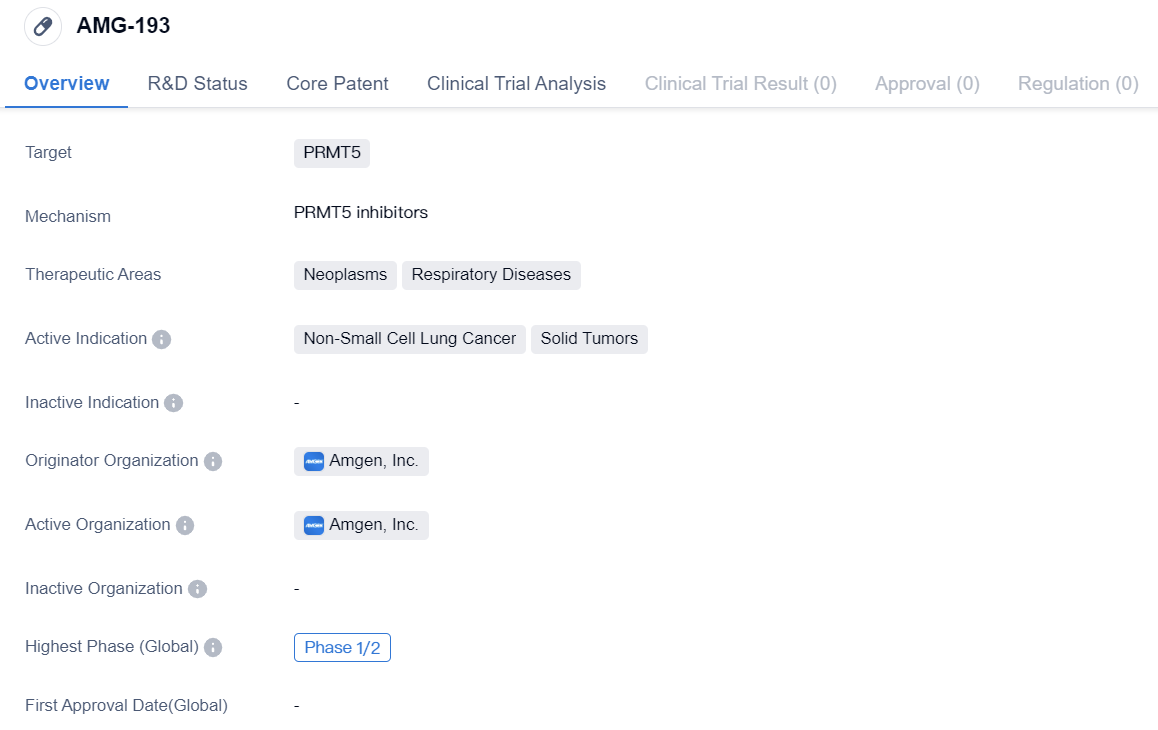
PRMT5 inhibitors entering PhaseⅠ/Ⅱclinical studies: AMG-193
AMG-193 is a small molecule drug developed by Amgen, Inc. It falls under the therapeutic areas of neoplasms and respiratory diseases, specifically targeting PRMT5. The drug is primarily indicated for the treatment of non-small cell lung cancer and solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Amgen, Inc. is the originator organization behind AMG-193. Being a small molecule drug, AMG-193 is designed to interact with PRMT5, a target protein involved in various cellular processes. By specifically targeting PRMT5, the drug aims to inhibit its activity and potentially disrupt the growth and survival of cancer cells in non-small cell lung cancer and solid tumors.
Neoplasms, or abnormal tissue growth, encompass a wide range of cancers. Non-small cell lung cancer is one of the most common types of lung cancer, accounting for approximately 85% of all cases. Solid tumors, on the other hand, refer to abnormal masses of tissue that can occur in various organs, such as the breast, colon, or prostate.
The current development stage of AMG-193, Phase 1/2, suggests that the drug has undergone initial testing in a small group of patients to evaluate its safety, dosage, and potential efficacy. This phase typically involves assessing the drug's pharmacokinetics, side effects, and preliminary therapeutic effects. The fact that AMG-193 has obtained IND approval in China indicates that it has met the necessary regulatory requirements to proceed with clinical trials in the country.
In summary, AMG-193 is a small molecule drug developed by Amgen, Inc. that targets PRMT5. It is indicated for the treatment of non-small cell lung cancer and solid tumors. The drug has reached Phase 1/2 on a global scale and has obtained IND approval in China, allowing for further clinical trials in the country.