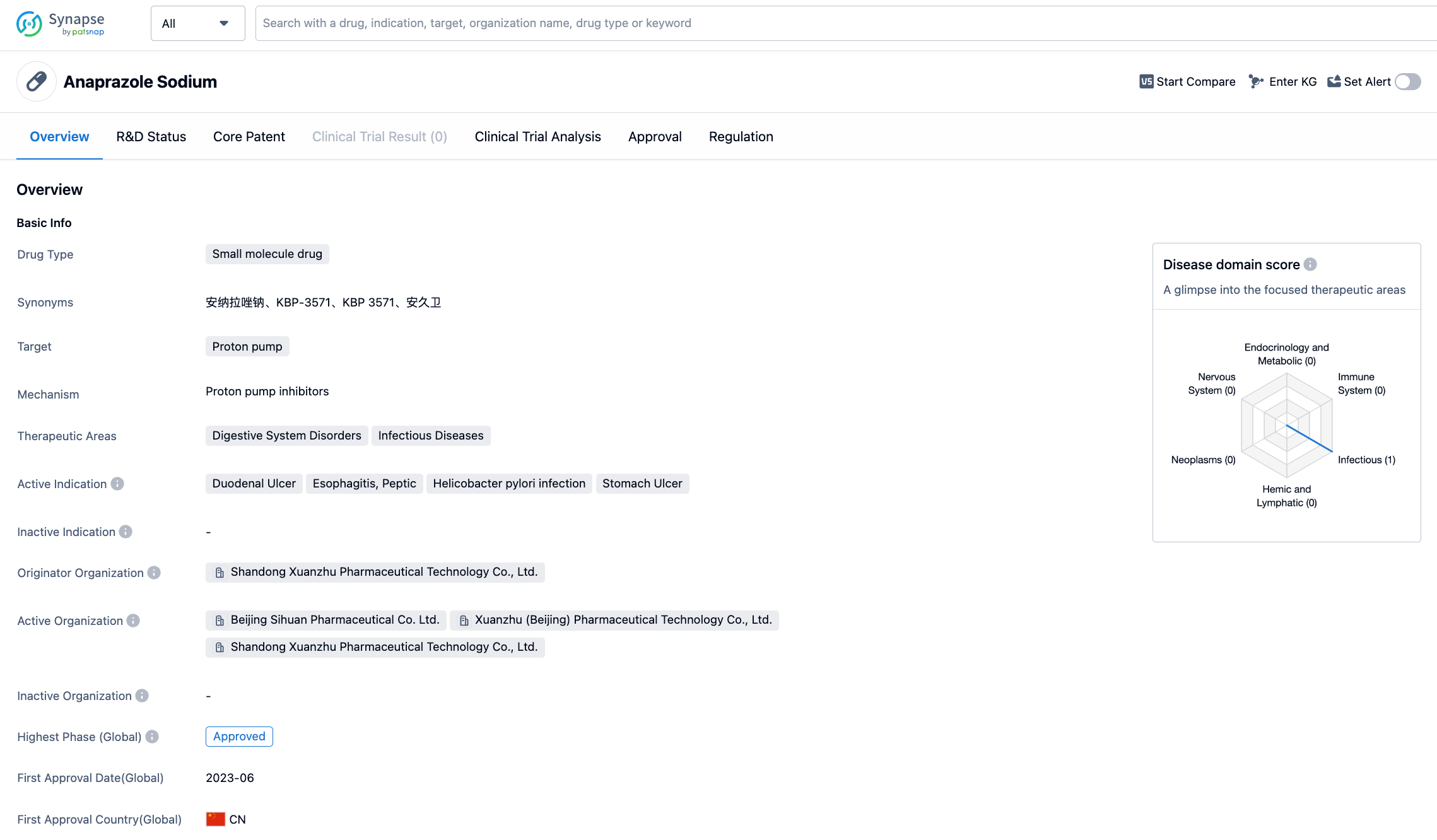
Anaprazole Sodium——Next Generation Proton Pump Inhibitors
Anaprazole Sodium is a small molecule proton pump inhibitor (PPI) developed by Beijing Sihuan Pharmaceutical Co. Ltd., approved by NMPA on June 21, 2023, for the treatment of duodenal ulcers.
Anaprazole Sodium is PPI, a benzimidazole compound, which can inhibit gastric acid secretion by inhibiting H+- K+- ATP enzymatic activity of parietal cell and reducing proton transport capacity.
Efficacy
In a multicenter, randomized, double blind, double simulated, and positive drug parallel controlled phase III study (NCT04215653), patients with duodenal ulcers were randomly treated with Anaprazole Sodium+placebo or Rabeprazole+placebo in a 1:1 ratio (once daily for 4 weeks). The primary efficacy endpoint was the 4-week ulcer healing rate evaluated through blind independent review. The secondary endpoint is the proportion of patients with overall and individual improvement in duodenal ulcer symptoms at 4 weeks. This study included 448 patients (Anaprazole Sodium, n=225; Rabeprazole, n=223). The 4-week healing rates of Anaprazole Sodium and Rabeprazole were 90.9% and 93.7%, respectively, indicating that Anaprazole Sodium has no adverse effect on Rabeprazole. The overall symptom improvement rates of duodenal ulcers were 90.9% and 92.5%, respectively, and the improvement rates of individual symptoms in each group were similar. There was no significant difference in the cure rate between the two groups.
Safety
The incidence of sudden adverse events in the treatment of Anaprazole Sodium (72/220, 32.7%) and Anaprazole Sodium (84/219, 38.4%) is similar.
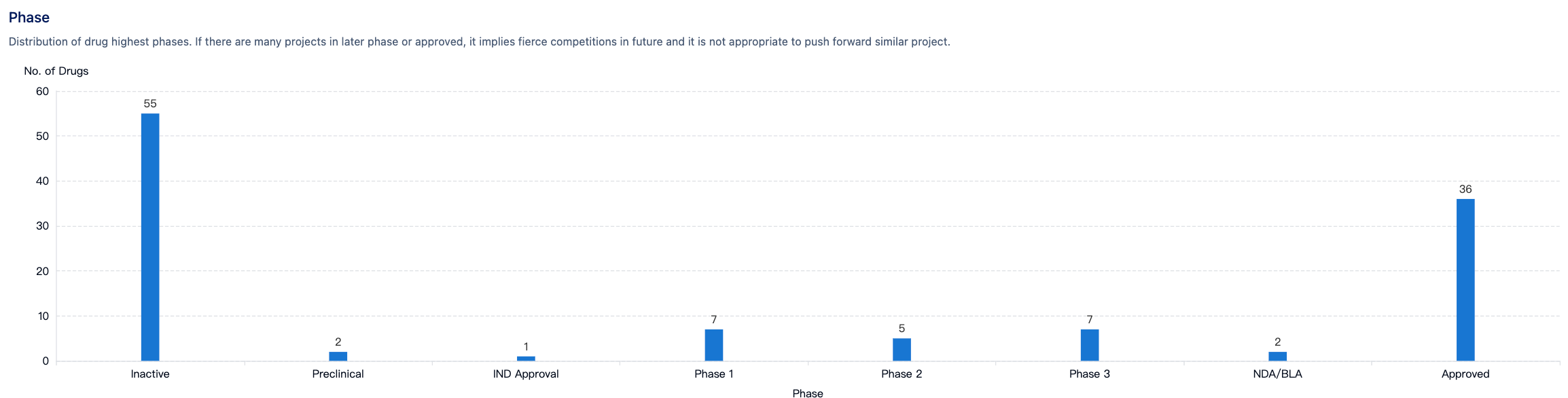
Competitive Landscape
According to “Synapse”, there are 82 PPI drugs, of which 36 have been approved, and the competitive environment is not severe. For more information, please click on the image link below.