Targeting the Siglec-9 Immune Checkpoint: A New Approach to Enhance Immunotherapy
Glioblastoma (GBM) is an extremely aggressive primary brain tumor. Currently, the standard treatment involves neurosurgery, adjuvant radiotherapy, and chemotherapy using temozolomide (TMZ). Despite this rigorous regimen, the likelihood of disease recurrence remains high, and survival rates post-recurrence are dismally low. Consequently, there's a pressing demand for novel treatment tactics for GBM.
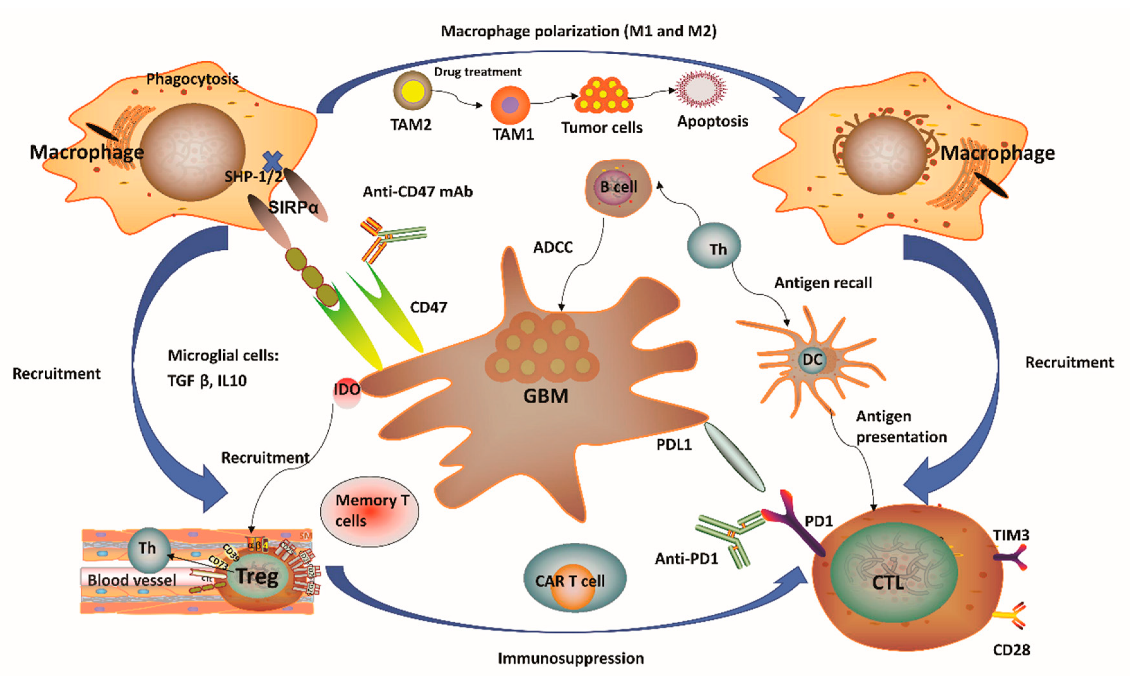
Immunotherapy has marked significant milestones in multiple cancer types. Unfortunately, GBM patients' response has been limited. This limitation can be attributed to the unique immunosuppressive microenvironment of GBM, characterized by a high population of tumor-associated macrophages (TAMs). TAMs interact uniquely with T cells, influencing their activation state, functional anti-tumor effects, and fostering tumor growth for pro-tumor effects. Efforts to regulate TAMs to enhance anti-tumor immune responses have been largely unsatisfactory, possibly due to the absence of ideal TAM targets.
The new target on TAMs
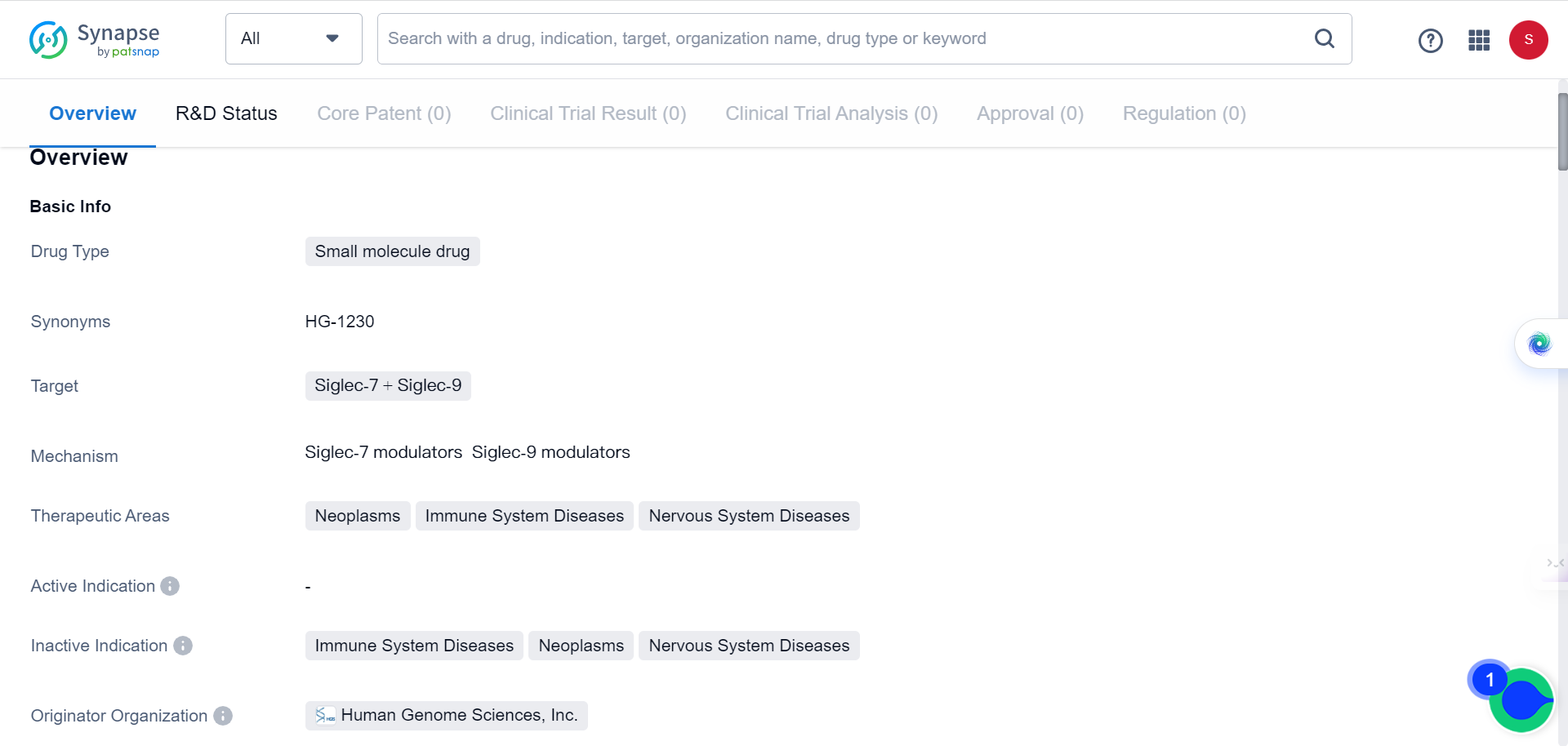
Recently, a study published in Nature Cancer identified a molecule called Siglec-9 that acts as an immune checkpoint molecule on the surface of TAMs in GBM, significantly affecting the tumor's immune environment and immunotherapy response. Siglec-9 is a relatively new target for the pharmaceutical industry, as evidenced by the fact that there is only one small molecule drug targeting it listed in the Synapse database:
In the Nature Cancer study, the research team analyzed samples from 24 glioblastoma (GBM) patients with primary, recurrent, and new adjuvant therapy with anti-PD-1 antibodies. They used single-cell RNA sequencing and spatial transcriptome analysis to find that patients who did not respond to anti-PD-1 treatment had many tumor-associated macrophages (TAMs) in the tumor with high expression of the Siglec-9 gene. Although anti-PD-1 treatment was found to change the tumor immune environment in some GBM patients, it could not change the overall immunosuppressive state of the intra-tumor environment, especially the immunosuppressive function of Siglec-9+ TAMs.
The research team further identified two types of Siglec-9+ TAMs: Siglec-9+ MARCO+ TAMs and Siglec-9+ SEPP1+ TAMs. They found that these cells were differentiated from CD14+ monocytes that migrated into the tumor and differentiated in a stepwise manner. The team also discovered that knocking out SiglecE (the mouse functional homolog of human Siglec-9) resulted in a significant increase in the number of activated T cells and a decrease in tumor growth. This suggests that Siglec-9 on TAMs plays a crucial role in restricting T cell priming and promoting tumor growth.
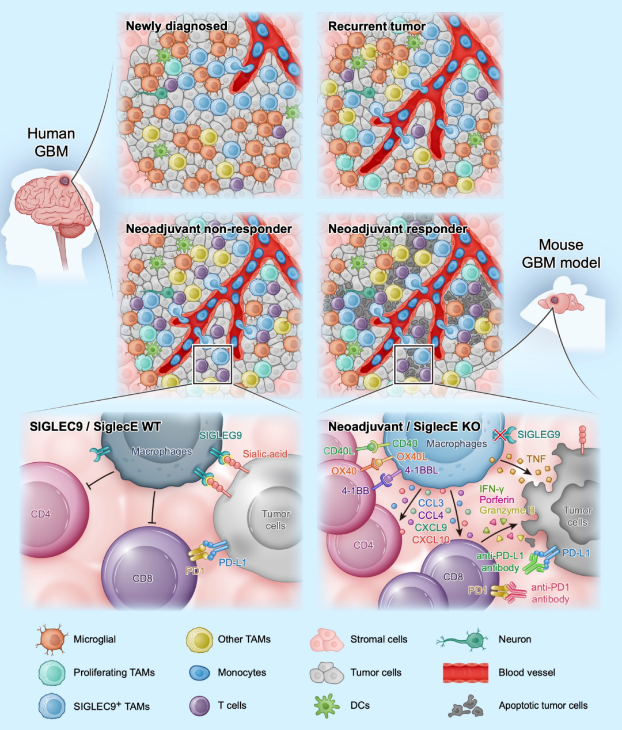
In addition, treatment with an anti-Siglec-9 antibody combined with anti-PD-1 antibody could significantly improve the response rate of GBM patients to immunotherapy, suggesting that Siglec-9 may be a potential target for improving the efficacy of immunotherapy in GBM patients.
These findings provide new insights into the unique immunosuppressive microenvironment of GBM and identifies Siglec-9 as a potential target for improving the efficacy of immunotherapy in GBM patients. However, further studies are needed to confirm these findings and to evaluate the safety and efficacy of anti-Siglec-9 therapy in GBM patients.
The sialic acid/Siglec-9 axis
Apart from TAMs, the expression of Siglec-9 on other immune cells also presents challenges. Another study, published in the journal Frontiers in Immunology, showed that sialic acid regulates immune evasion by engaging inhibitory Siglec receptors on myeloid and other immune cells. Inhibitory Siglecs have immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their intracellular domain, which become phosphorylated upon ligand binding and trigger immunosuppressive signaling.
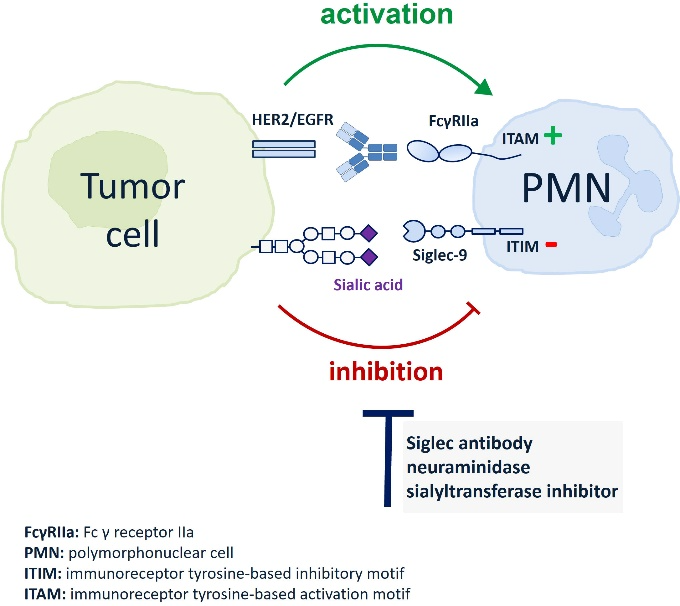
The researchers found that neutrophils express Siglec-9, and that EGFR and HER2 positive breast tumor cells express ligands for Siglec-9. Treatment of tumor cells with neuraminidases or a sialyl transferase inhibitor significantly reduced binding of a soluble recombinant Siglec-9-Fc fusion protein, while EGFR and HER2 expression remained unchanged.
The cytotoxic activity of neutrophils driven by therapeutic EGFR or HER2 antibodies in vitro was increased by blocking the sialic acid/Siglec interaction, either by reducing tumor cell sialylation or by a Siglec-9 blocking antibody containing an effector silenced Fc domain. In an in vivo study using a short-term xenograft mouse model, the improved therapeutic efficacy of EGFR antibodies against tumor cells depleted of sialic acid, achieved through the use of a sialyltransferase inhibitor, was confirmed. This was in comparison to the efficacy observed in untreated cells.
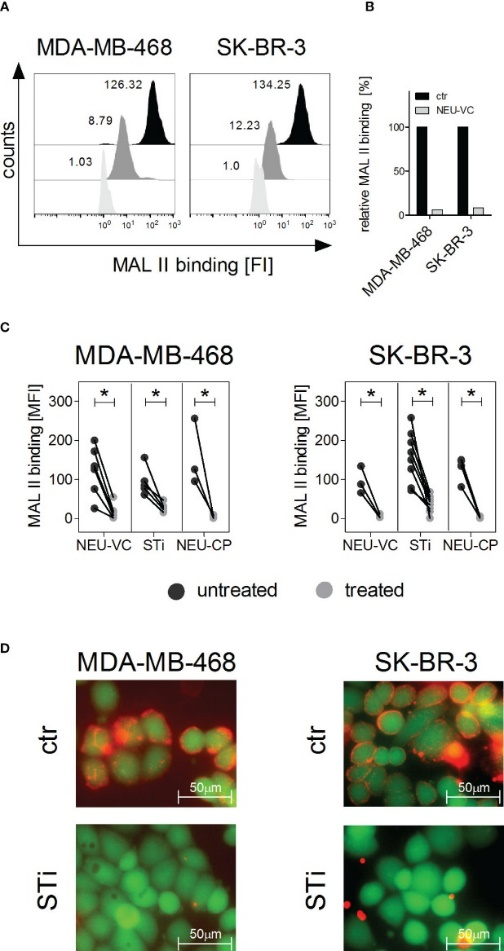
The team's findings demonstrated that sialic acid/Siglec interactions between tumor cells and myeloid cells can impair antibody-dependent tumor cell killing, and that Siglec-9 on polymorphonuclear cells (PMN) is critically involved. Considering that PMN are often a highly abundant cell population in the tumor microenvironment, Siglec-9 constitutes a promising target for myeloid checkpoint blockade to improve antibody-based tumor immunotherapy.
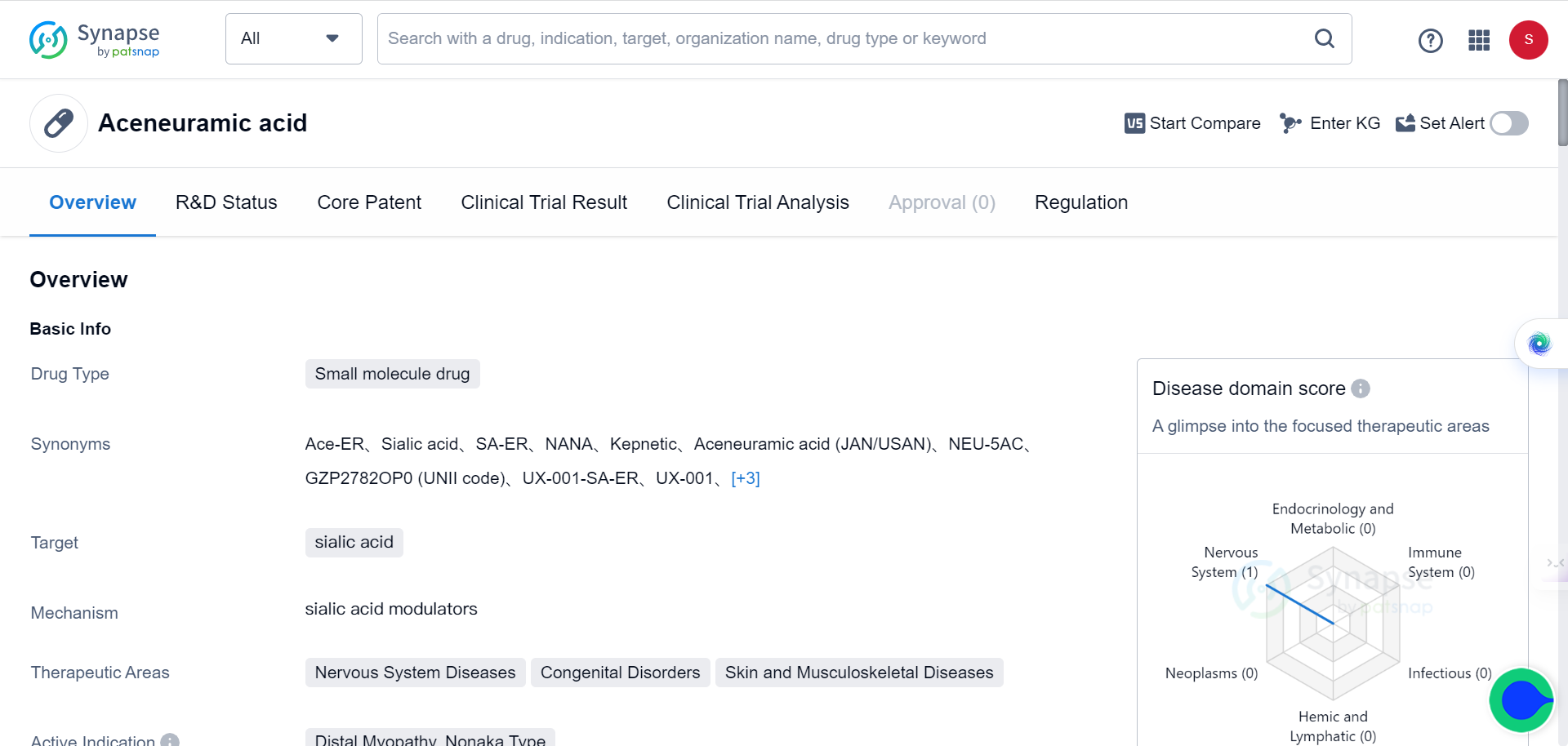
Similar to the situation with Siglec-9, there’s also only one entry for drug targeting sialic acid in Synapse database:
The rarity of drugs targeting the newly-discovered sialic acid/Siglec-9 axis in cancer treatment may signify a new frontier with abundant opportunities opened up. The studies discussed here provide a foundation for the development of novel approaches to improve the efficacy of cancer immunotherapy.

References:
1.Mei, Y., Wang, X., Zhang, J. et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat Cancer (2023).
2.Lustig M, Chan C, Jansen JHM, et al. Disruption of the sialic acid/Siglec-9 axis improves antibody-mediated neutrophil cytotoxicity towards tumor cells. Front. Immunol. 14:1178817(2023).