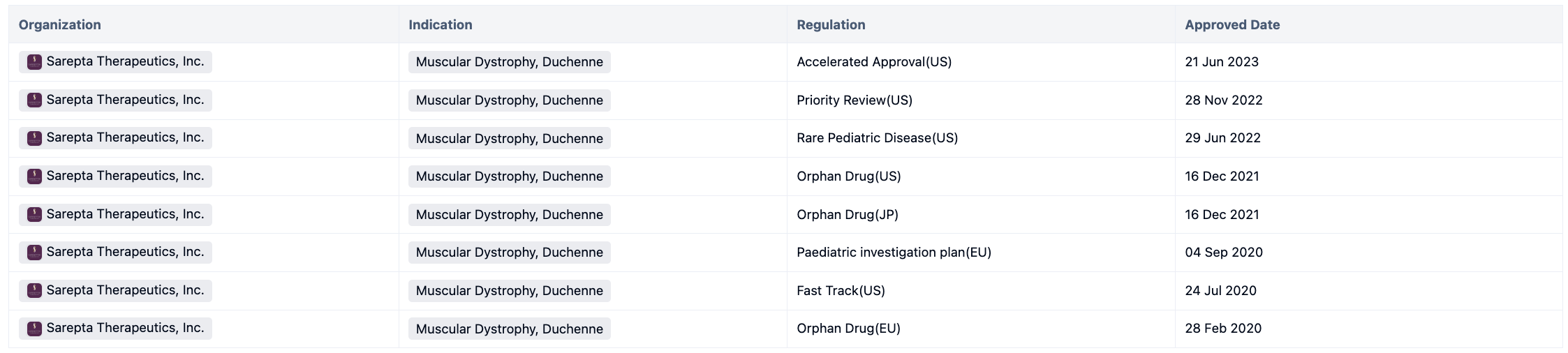
Delandistrogene moxeparvovec - The first DMD Gene Therapy
Delandistrogen moxeparvovec, also known as SRP-9001, trade name ELEVIDYS, is a kind of Adeno-associated virus gene therapy targeting micro dystrophin. This drug was developed by Nationwide Children's Hospital and was approved by the FDA on June 21, 2023, for the treatment of pediatric patients 4 through 5 years of age with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene who do not have a pre-existing medical reason preventing treatment with this therapy. Elevidys was approved through the Accelerated Approval pathway.
Elevidys is the first gene therapy for the treatment of pediatric patients 4 through 5 years of age with DMD. This drug has obtained multiple regulations during its development process. For more information, please click on the image link below.
DMD is a rare and serious genetic condition which worsens over time, leading to weakness and wasting away of the body’s muscles. The disease occurs due to a defective gene that results in absence of dystrophin, a protein that helps keep the body’s muscle cells intact. As a result of this genetic defect, individuals with DMD may have symptoms such as trouble walking and running, falling frequently, fatigue, learning disabilities/difficulties, heart issues as a result of impact on heart muscle functioning, and breathing problems due to weakening of respiratory muscles involved in lung function. Symptoms of muscle weakness associated with DMD typically begin in childhood, often between 3 to 6 years of age.
Mechanism of Action
Elevidys is a recombinant gene therapy designed to deliver into the body a gene that leads to production of Elevidys micro-dystrophin, a shortened protein (138 kDa, compared to the 427 kDa dystrophin protein of normal muscle cells) that contains selected domains of the dystrophin protein present in normal muscle cells.
Efficacy and Safety
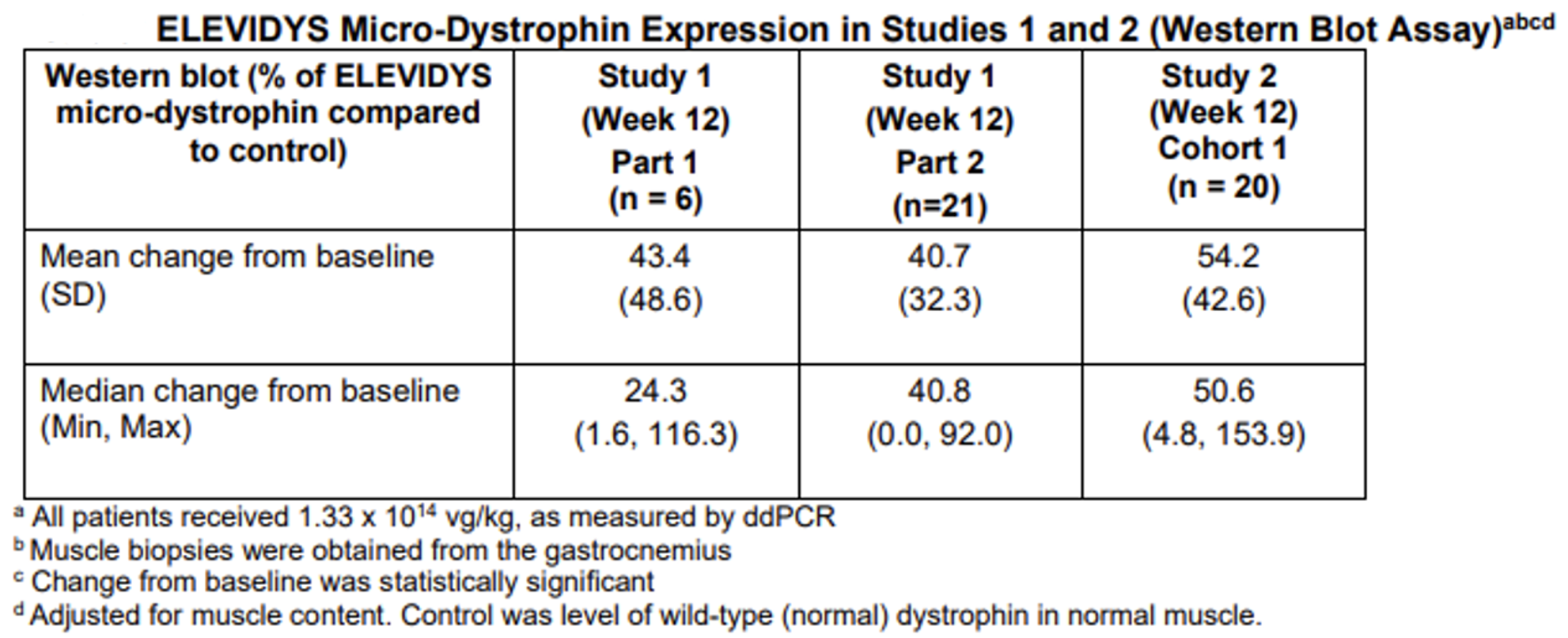
The accelerated approval of Elevidys was based on data from the randomized clinical trial that established that Elevidys increased the expression of the Elevidys micro-dystrophin protein observed in Elevidys-treated individuals aged 4 to 5 years with DMD.
The most commonly reported side effects by individuals who received Elevidys were vomiting, nausea, acute liver injury, pyrexia (fever) and thrombocytopenia (abnormally low platelet count in the blood). Patients’ liver function should be monitored before treatment with Elevidys, and weekly for the first three months after treatment. Patients given Elevidys may also be at risk for severe immune-mediated myositis (muscle inflammation). Additionally, myocarditis (inflammation of heart muscle) and elevations of troponin-I (a heart protein found in the blood after heart muscle injury) have been observed following use of Elevidys in clinical trials. Troponin-I levels should be monitored before administration of Elevidys and weekly for the first month after treatment.
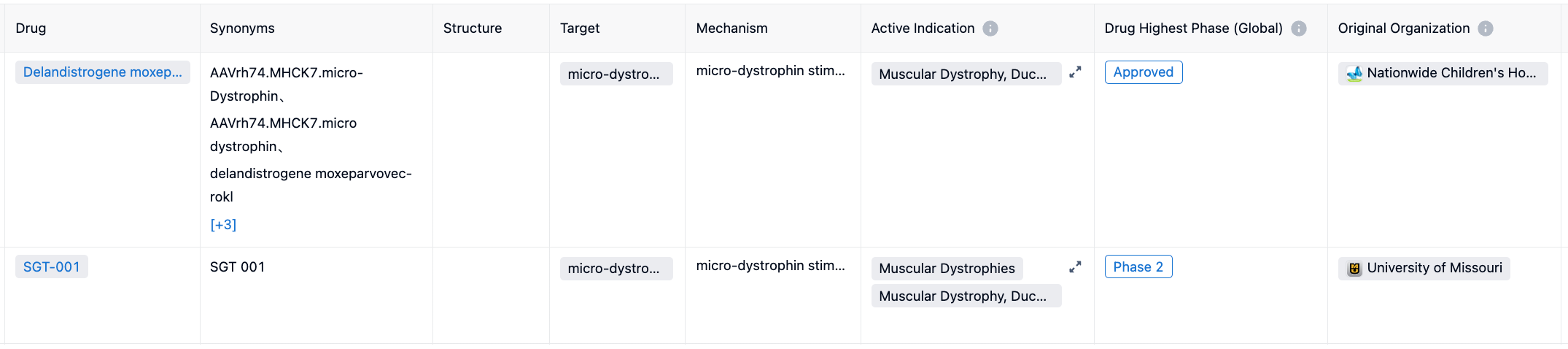
Competitive Landscape
Most current treatment approaches address the symptoms of the disease, but not its underlying genetic cause. Treatments include corticosteroid medications to slow down the progression of muscle weakness, stretching and exercise programs, and use of equipment such as braces or a wheelchair as walking becomes more difficult. Antisense oligonucleotides (ASOs) facilitate exon skipping for specific DMD gene mutations, but the ASOs can only address a minority of the gene mutations and require repeated administration. The approval addresses an urgent unmet medical need and is an important advancement in the treatment of DMD. Its potential competitor is SGT-001, currently in clinical phase 2. For more information, please click on the image link below.