Aprocitentan: The Hypertension Drug Once Rejected by J&J Now Secures FDA Approval for Market Release
On March 20th, the Swiss pharmaceutical company Idorsia announced that the U.S. FDA has approved TRYVIO™ (aprocitentan) for use in combination with other antihypertensive medications to treat adult hypertension, in order to lower blood pressure levels in the adult patient population whose blood pressure has not been effectively controlled.

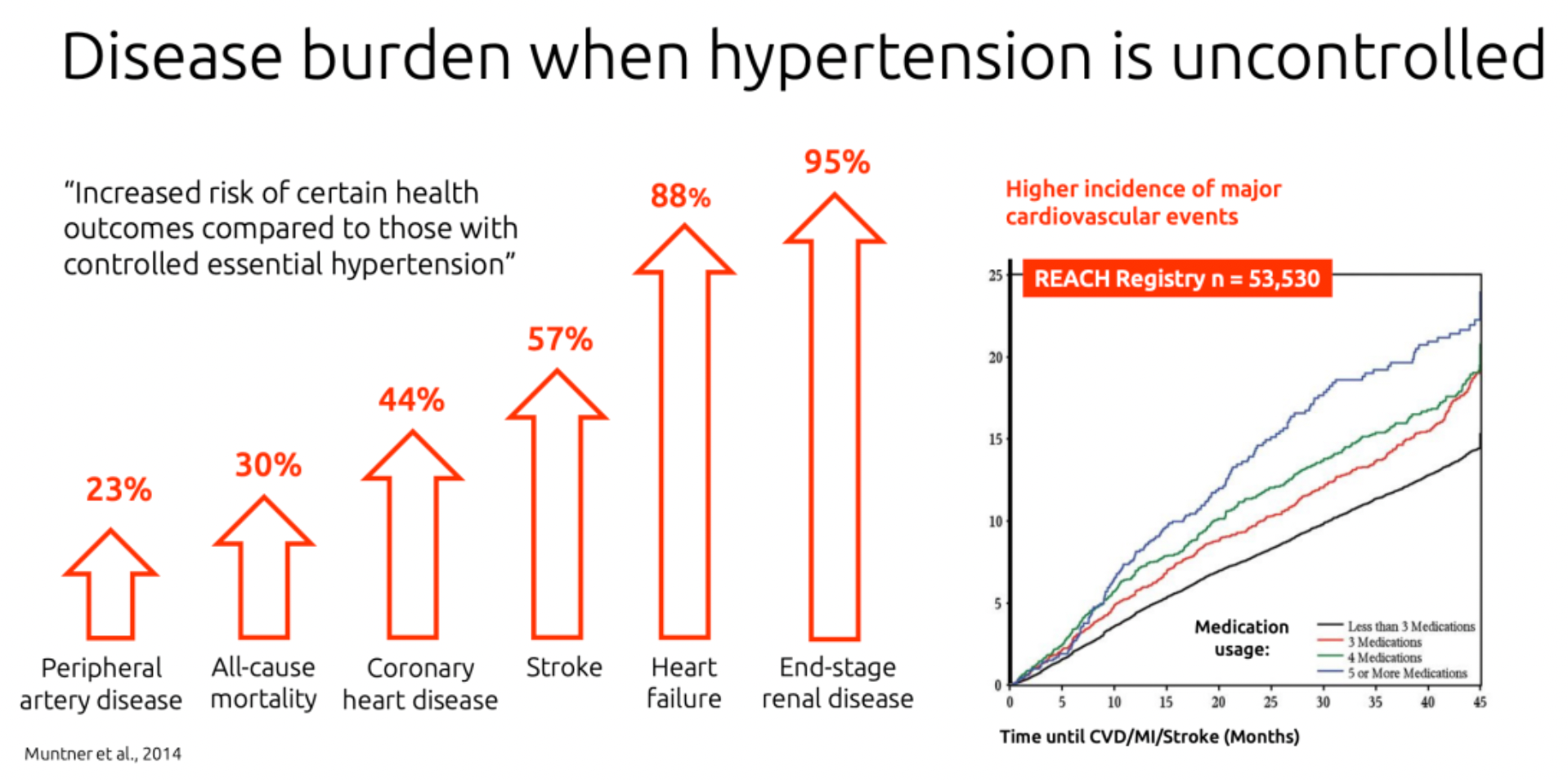
Hypertension is a common chronic condition characterized by persistently high blood pressure readings (140/90 mmHg or higher). Lowering high blood pressure can reduce the risk of fatal and non-fatal cardiovascular events, with stroke and myocardial infarction being the primary concerns.
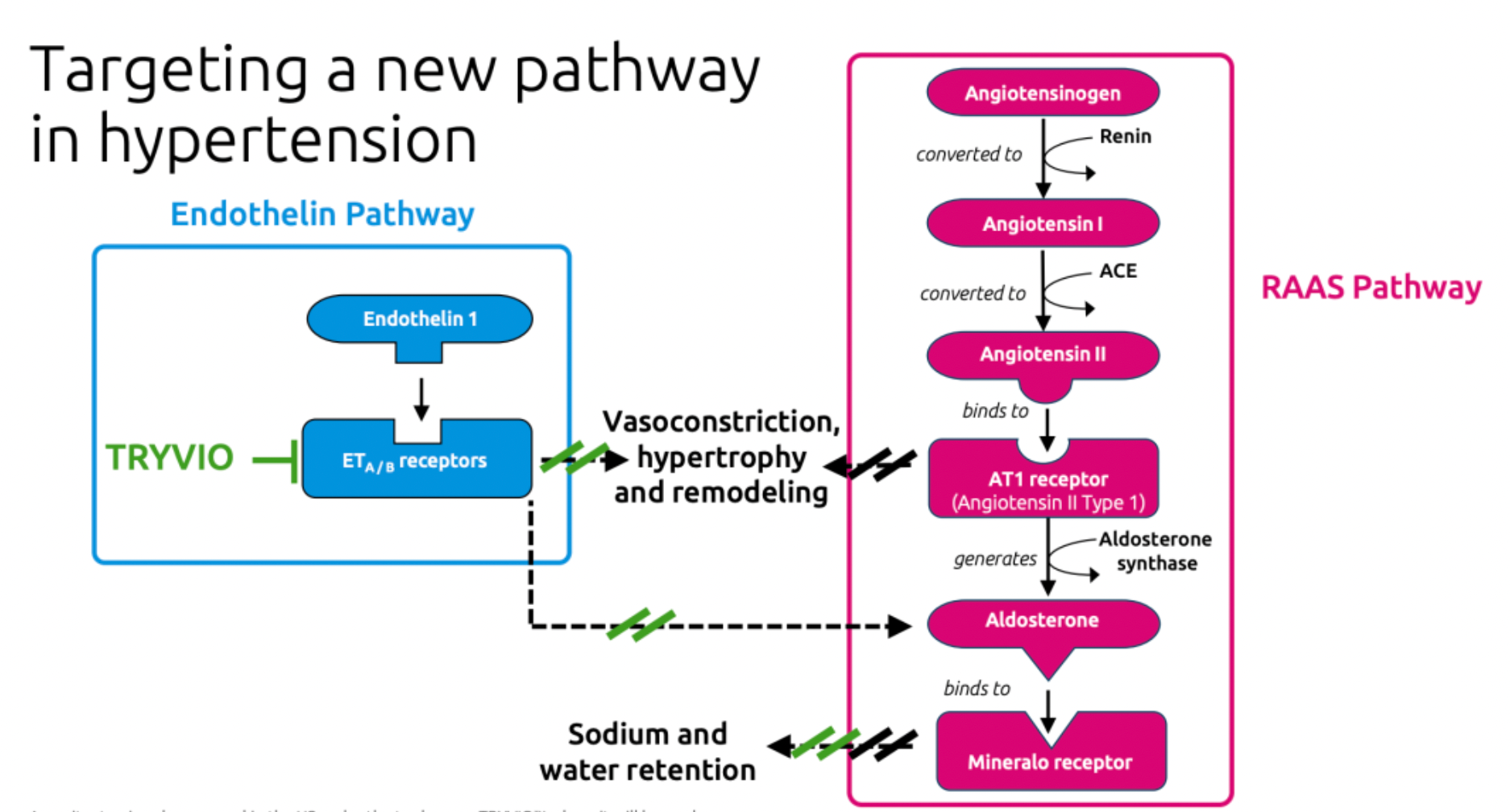
·TRYVIO acts by targeting endothelin-1 (ET-1), which is a potent vasoconstrictor closely associated with the mechanisms leading to hypertension. It mitigates the detrimental effects of ET-1 by preventing its binding to ETA and ETB receptors, thereby reducing the vasoconstrictive, fibrotic, cellular proliferative, and inflammatory responses induced by ET-1.
·TRYVIO tablets are required to be taken only once daily and can be ingested with or without food.
·TRYVIO comes with a black box warning, indicating its teratogenic toxicity. It is contraindicated for use in pregnant women. Effective contraceptive measures must be taken before starting treatment, throughout the course of treatment, and for one month after discontinuation.
·Warnings and precautions associated with TRYVIO include risks of hepatotoxicity, fluid retention, decrease in hemoglobin, and reduced sperm count. Common adverse reactions comprise edema/fluid retention and anemia.
The following is a retrospective review of TRYVIO's R&D milestones over the past 10 years at various time points:
2023: Submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA).
2022: Filed a New Drug Application (NDA) with the U.S. FDA, and in the same year, presented the work as a late-stage breakthrough study at an American Heart Association (AHA) meeting, with relevant Phase III clinical trial data published in the prestigious medical journal The Lancet.
2022: Successfully completed Phase III clinical studies, demonstrating the drug's safety and efficacy in the targeted patient population.
2018: Initiated Phase III clinical trial, a crucial step in validating the drug's therapeutic effectiveness and safety in a large population.
2017: The dose-response study yielded positive results, confirming the pharmacodynamic response of Aprocitentan at different dosages, laying the foundation for subsequent research.
2015: Began Phase II dose-response relationship studies aimed at further exploring the optimal dosage range of the drug and its response in humans.
2014: Launched Phase I clinical trial program, marking the first step for Aprocitentan to move from laboratory to human clinical testing, primarily to assess the drug's safety, tolerability, and preliminary pharmacokinetic characteristics in humans.
Hypertension Medication Returned by Johnson & Johnson
On January 26, 2017, Johnson & Johnson announced a proposal to acquire the Swiss pharmaceutical company Actelion in an all-cash tender offer, at a price of $280 per share (totaling $30 billion in cash) for all of Actelion's outstanding shares.
As part of the transaction, Actelion will spin off its drug research and early-stage clinical development assets into a newly formed Swiss biopharmaceutical company called Idorsia. Idorsia will be listed on the Swiss Stock Exchange, and Johnson & Johnson will initially hold a 16% stake in the newly established R&D company.
In November 2017, the newly formed Idorsia announced a collaboration with Janssen Biotech, a subsidiary of Johnson & Johnson, focusing on Aprocitentan. As part of the agreement, Idorsia will receive about $230 million in milestone payments; Idorsia and Janssen Biotech will share the development costs of aprocitentan during the Phase III clinical trials; and Idorsia will be entitled to royalty fees on potential future net sales.
On September 6th, 2023, Idorsia announced that the company had reached an agreement with Janssen Biotech, a subsidiary of Janssen Pharmaceuticals, to reacquire the rights to Aprocitentan.
It was reported that, although Phase 3 clinical trial results of the drug showed a significant reduction in blood pressure compared to placebo, they also revealed an issue where 18% of patients on high doses experienced mild to moderate fluid retention. Johnson & Johnson deemed that the drug might not meet their commercial development objectives and therefore decided to return the rights.
In return, Idorsia will pay Janssen Biotech approximately 306 million Swiss francs, contingent upon the approval of Aprocitentan's marketing application by the US FDA and the European EMA. Additionally, Idorsia is currently exploring the best approach to maximize the value of aprocitentan."
Aprocitentan is a novel oral dual endothelin receptor antagonist currently being developed by Idorsia Pharmaceuticals. It effectively inhibits the binding of ET-1 to both ETA and ETB receptors. It is reported that the potential for drug-drug interactions with Aprocitentan is low, and its mechanism of action is highly suitable for the pathophysiology of resistant hypertension.
The Latest Clinical Data on Aprocitentan
In November 2022, the team from Idorsia and Johnson & Johnson published an article in The Lancet journal, presenting Phase 3 clinical study data of aprocitentan for the treatment of resistant hypertension. The results indicated that aprocitentan was well-tolerated in patients with resistant hypertension, showing a superior blood pressure-lowering effect compared to placebo at week 4 and sustained efficacy at week 40.

The data demonstrated that among the 730 patients enrolled in the study, the least squares mean changes in systolic blood pressure after the first 4 weeks of treatment were -15.3 mm Hg for aprocitentan 12.5 mg, -15.2 mm Hg for aprocitentan 25 mg, and -11.5 mm Hg for placebo. The differences compared to placebo were -3.8 mm Hg and -3.7 mm Hg, respectively. Four weeks after discontinuation of the medication, systolic blood pressure significantly increased by 5.8 mm Hg compared to placebo.
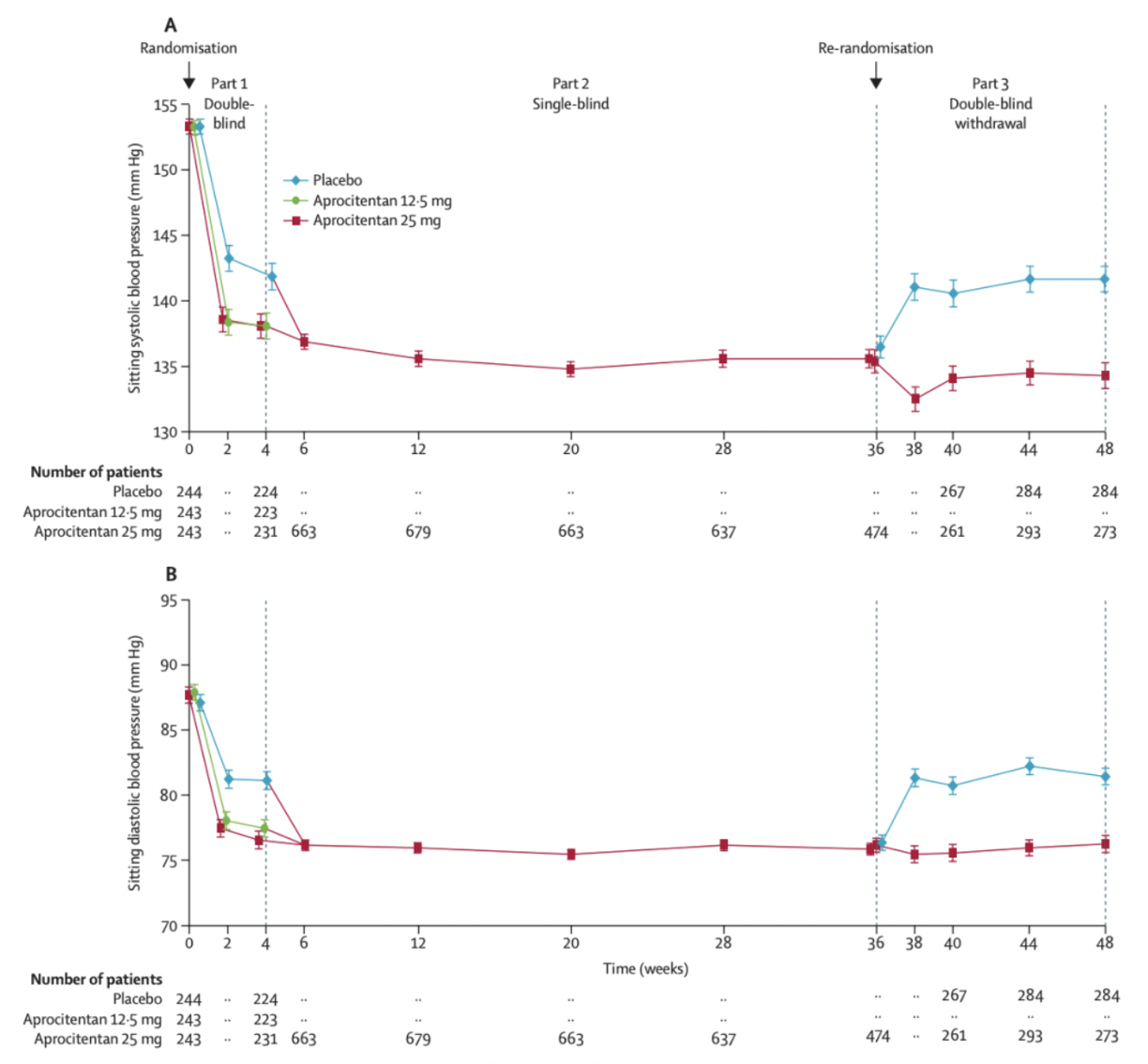
The most common adverse events were mild to moderate edema or fluid retention, leading to discontinuation of aprocitentan treatment in 7 patients. There were 11 cases of sudden death during the trial, which the researchers considered unrelated to the treatment.
Based on this positive Phase 3 clinical data, Idorsia and Johnson & Johnson submitted marketing authorization applications for Aprocitentan to the United States in December 2022 and to the European Union in January 2023.
About Endothelin (ET)
Endothelin (ET) is a potent peptide vasoconstrictor, discovered in 1988 by Japanese scientist Yanagisawa and colleagues. It regulates blood pressure through its actions on blood vessels, the heart, and kidneys.
The ET peptide family consists of three members: ET-1, ET-2, and ET-3. These ET peptides are each 21 amino acids in length and their overall structure is constrained by two disulfide bonds. The carboxyl-terminal sequence is essential for their activity.
Taking ET-1 as an example, the translated ET-1 exists in the form of a precursor. Under the action of endothelin-converting enzyme (ECE), it undergoes proteolytic processing from an inactive precursor to a mature peptide, providing a potential pathway for inhibiting the production and action of ET-1.
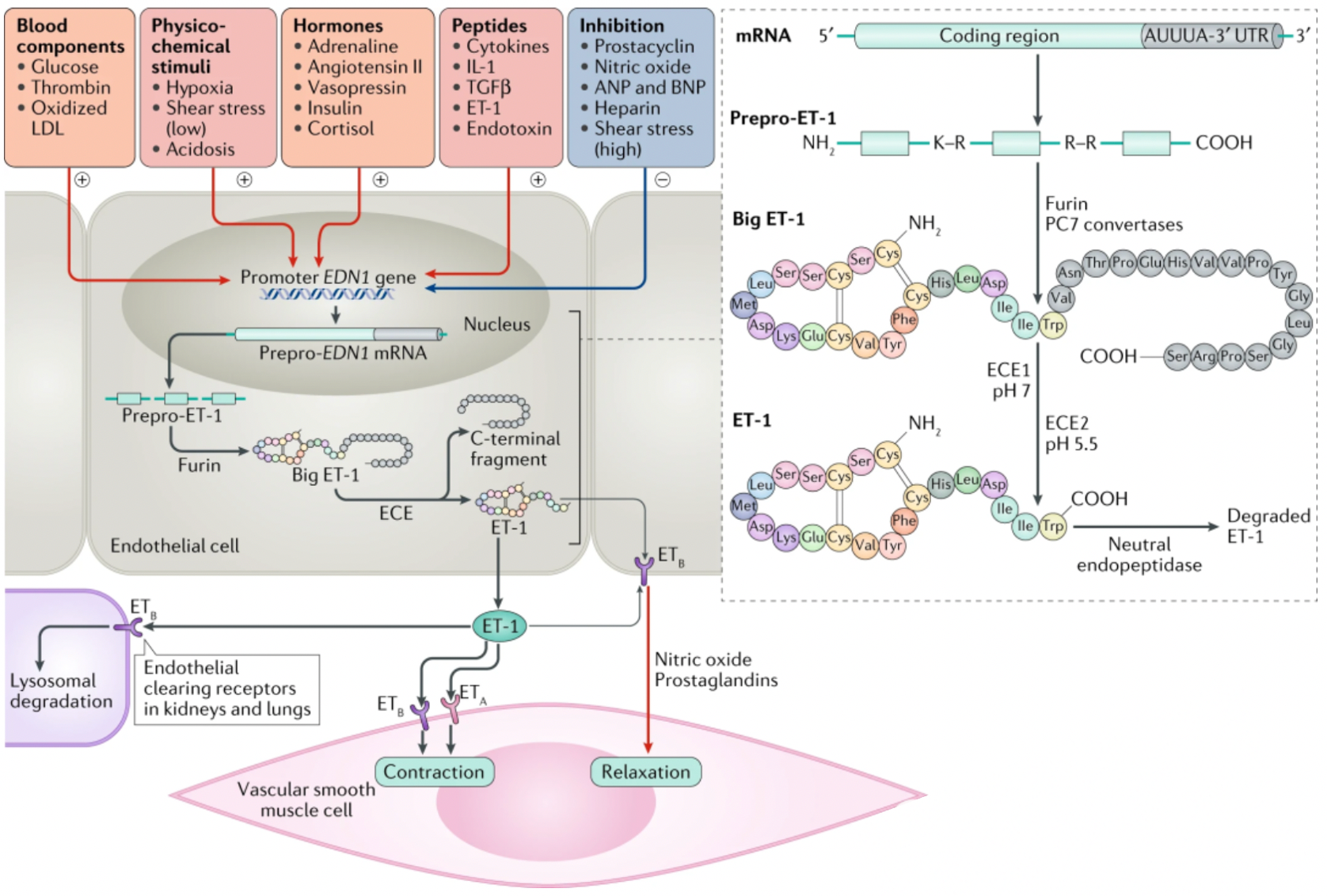
Humans have two types of ET receptors, the ETA receptor (ETA) has a higher binding affinity with ET-1 and ET-2 than with ET-3; the ETB receptor (ETB) has comparable binding affinity with all three isoforms.
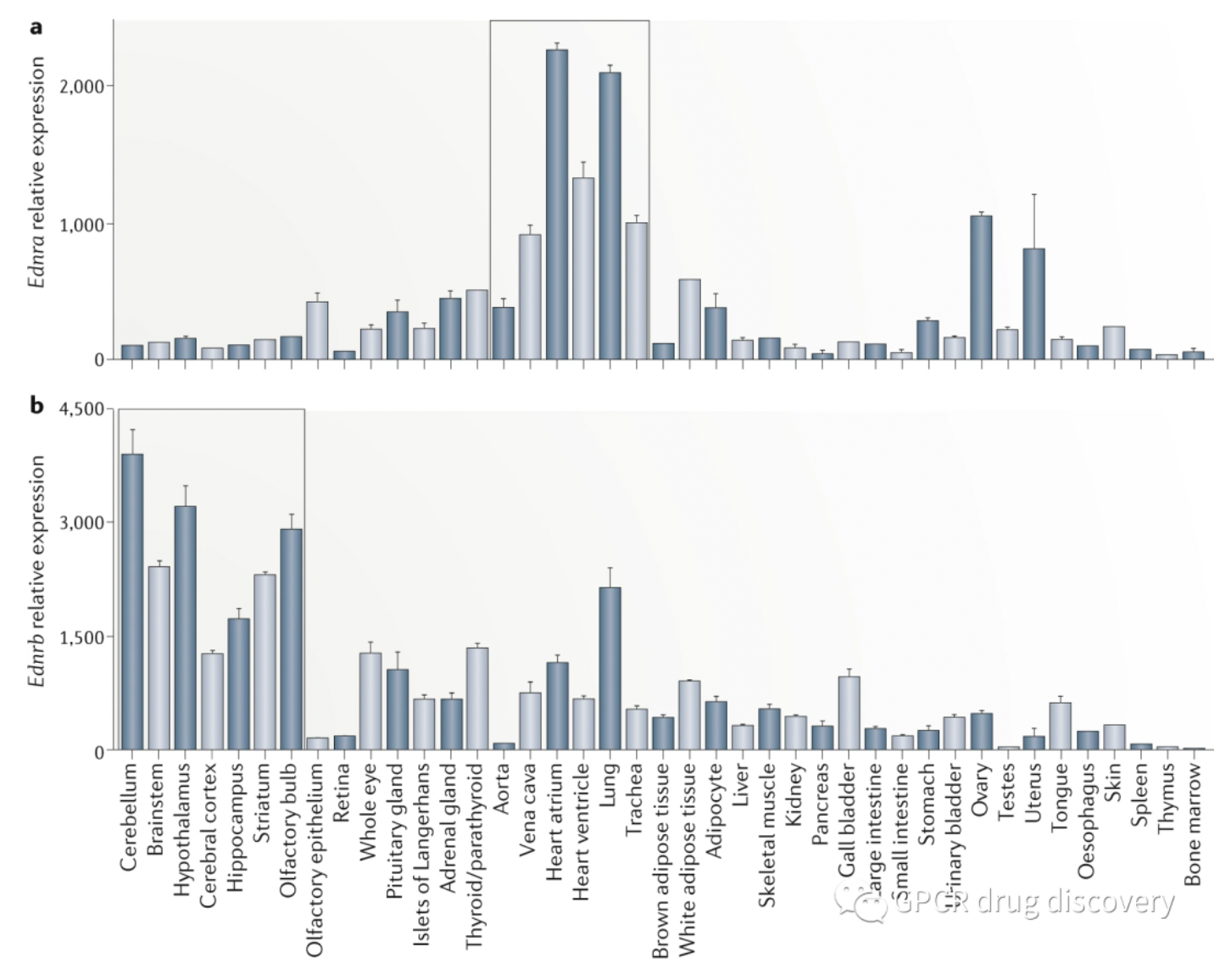
The figure below shows the relative mRNA expression levels of the ETA receptor and ETB receptor in 41 types of adult mouse tissues. The ETA receptor is highly expressed in the cardiovascular and pulmonary tissues, whereas the ETB receptor is highly expressed in the brain tissue.
About Endothelin Receptor Antagonists
Endothelin receptors are widely known for their involvement in the physiological regulation of vascular tone due to their potent vasoconstrictive action. Excessive secretion of endothelin in the lungs can lead to pulmonary arterial hypertension. Endothelin autoregulates itself through physical and chemical factors such as blood flow, mechanical stretch, or pH changes and triggers the production of growth factors.
Moreover, chronic endothelin stimulation has been shown to be associated with a variety of human cardiovascular, inflammatory, fibrotic, and neoplastic diseases. Additionally, endothelin may also play a role in conditions such as heart failure, renal insufficiency, septic shock, atherosclerosis, and hemorrhage-associated cerebrovascular diseases. Therefore, drugs that act on endothelin receptors may be beneficial in the treatment of a multitude of diseases.
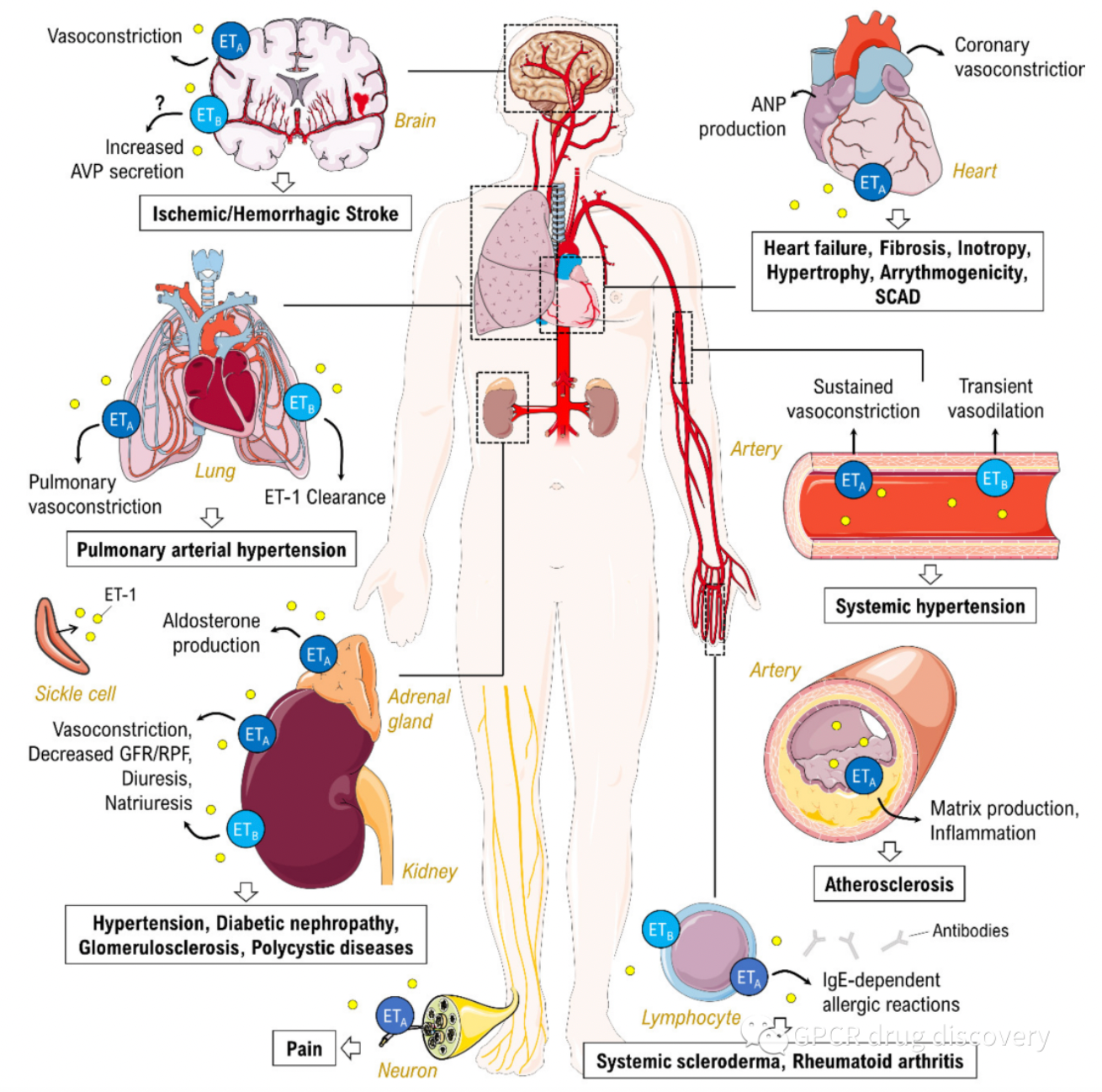
Currently, several companies are screening antagonists targeting the ETA and ETB receptors, including selective antagonists for the ETA receptor, selective antagonists for the ETB receptor, or dual antagonists. Multiple endothelin receptor antagonists have been approved for the treatment of this disease.
Current Status of Drug Development
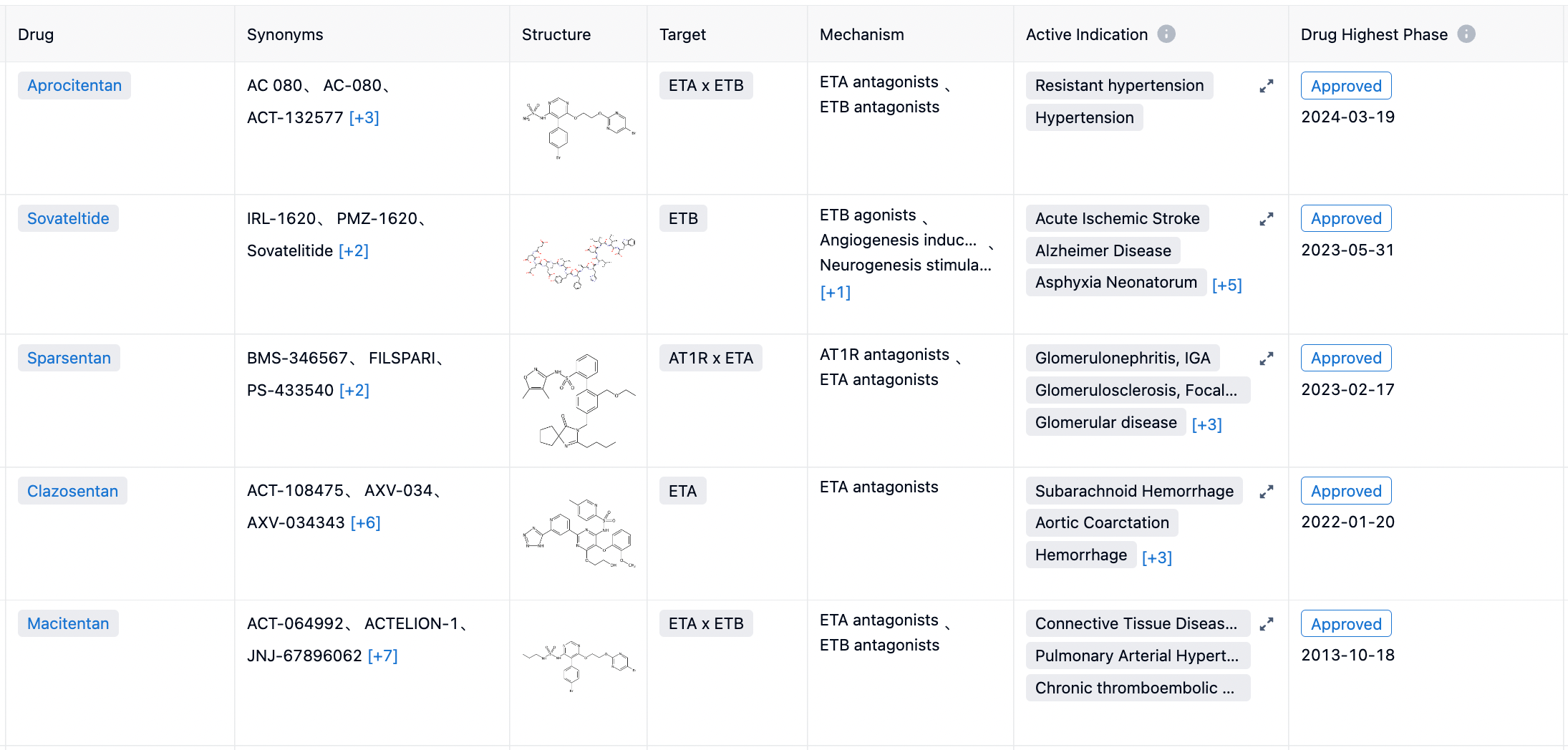
According to statistics from the Synapse database, there are a total of 5 endothein receptor antagonists (ETA or ETB antagonists) approved by the FDA, including Bosentan (2001, dual antagonist), Sitaxentan (2006, withdrawn), Ambrisentan (2007, ETA receptor selective), Macitentan (2013, dual antagonist), and Sparsentan (2023, ETA+AT1R dual antagonist).
In February 2023, a dual antagonist targeting the inner ETA receptor and angiotensin II (Ang II) type 1 receptor (AT1R) named Filspari (Sparsentan) was approved by the FDA for marketing. The pharmaceutical is indicated for adults with primary immunoglobulin A nephropathy who are at risk of rapid disease progression and aims to reduce proteinuria.
In May 2023, an ETA receptor selective agonist developed by the biopharmaceutical company Pharmazz, Sovateltide injectable solution, was approved for marketing in India for the treatment of patients with acute ischemic stroke.
On September 14, Pharmazz entered into a licensing agreement with the Indian pharmaceutical company Sun Pharma. Under this agreement, Sun Pharma will have the rights to sell Sovateltide in India under the brand name Tyvalzi™. Pharmazz will be entitled to upfront and milestone payments, including royalties.

Regarding Endothelin and Antitumor Drugs
Aside from the symptoms of hypertension, endothelin receptors have multiple functions related to fundamental cellular processes such as cell proliferation and apoptosis.
Aberrant expression of ET1 or overexpression of endothelin receptors or their associated signaling pathways can promote the occurrence and development of tumors through autocrine and paracrine mechanisms. These altered mechanisms can originate from genetic and epigenetic changes. In addition, a complex crosstalk network between ET1 signaling and other growth factor pathways propels tumor progression. This includes the interactions between endothelin receptors and receptors for epidermal growth factor and vascular endothelial growth factor.
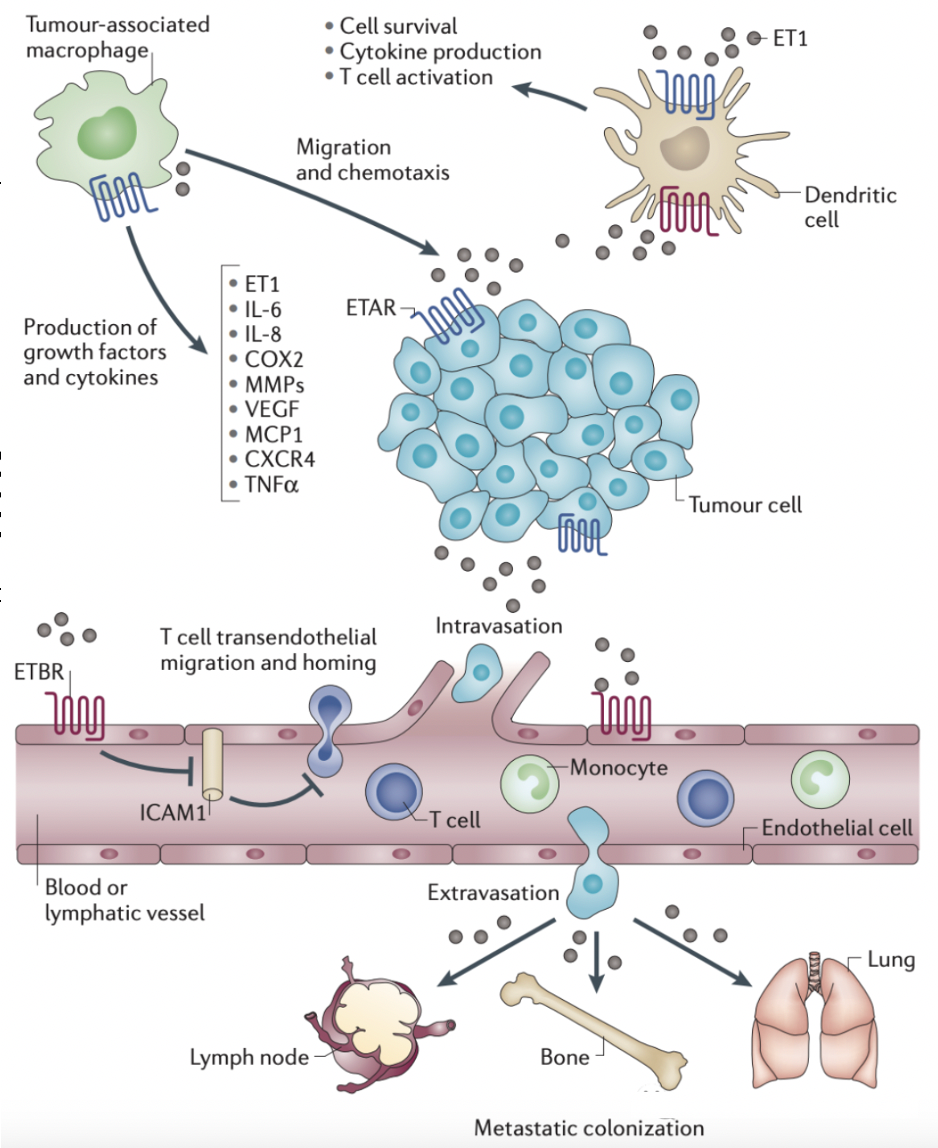
The ET1 signal can promote cell proliferation, survival, epithelial-mesenchymal transition (EMT), angiogenesis, immune cell responses, and drug resistance, with its mechanisms of action depending on specific contexts. Consequently, endothelin receptors have become key targets in cancer therapy.
The figure below shows the expression of endothelin receptors in some human malignancies.
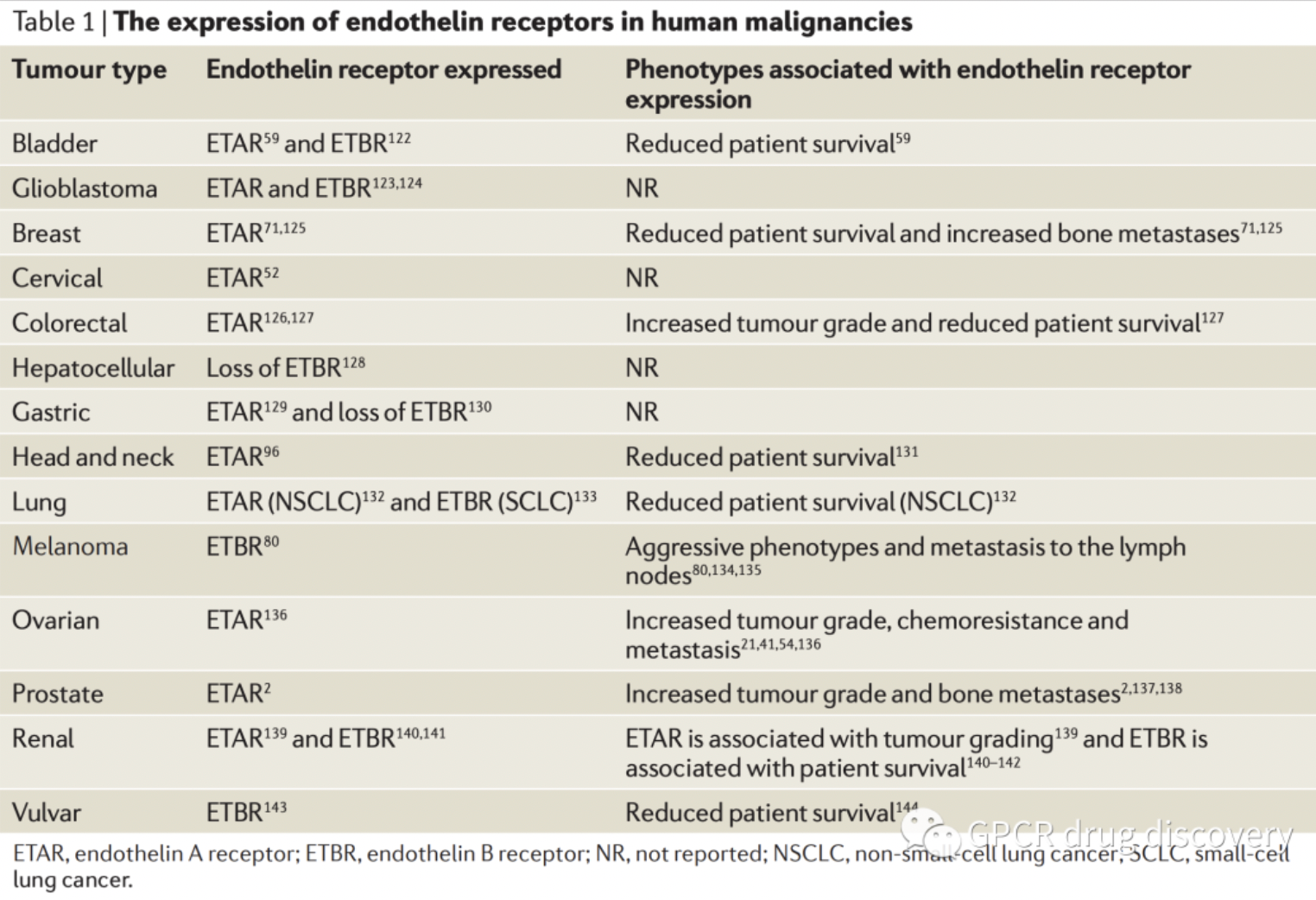
According to statistics from the Synapse database, there are currently several clinical candidate drugs globally targeting ETA or ETB receptors for research into various types of solid tumors. This includes the ETB receptor antagonist VRP-1620, developed by the University of Illinois for breast cancer research, and ENB Medical's selective ETB receptor small molecule antagonist ENB-003, both of which are in clinical research stages.
Chinese innovative pharmaceutical company Gmax Biopharm LLC is conducting research on GMA202, a bispecific M-Body molecule consisting of an antibody targeting the ETA receptor and an antibody fragment targeting CD3. Reportedly, GMA202 achieves a unique and precise tumor immunotherapy effect by pairing activated T cells with ovarian cancer cells that highly express the ETA receptor.
RG-7636 (DEDN6526A) is an ADC product developed by Genentech, which uses the ETB receptor as the mAb target linked to MMAE. Preclinical studies have shown that RG-7636 exhibits dose-dependent antitumor activity against xenografts expressing the ETB receptor. Phase 1 clinical trials have demonstrated that RG-7636 has antitumor activity in patients with melanoma.