AriBio Gains IND Approval from China's NMPA for Phase 3 Alzheimer's Trial, POLARIS-AD
AriBio Co., Ltd., a biotechnology firm focused on developing innovative therapies for neurodegenerative conditions, has revealed the approval of their Investigational New Drug application by the Center for Drug Evaluation of China's National Medical Products Administration. This approval allows for the commencement of the Phase 3 Polaris-AD trial for AR1001 in early Alzheimer's Disease in China starting May 11th, 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase 3 clinical trial will recruit early Alzheimer's Disease (AD) patients at up to 20 trial centers in China, with consistent eligibility criteria as in other participating countries. Key evaluation metrics include the Clinical Dementia Rating Scale – Sum of Boxes, Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Amsterdam-Activities of Daily Living Questionnaire, Geriatric Depression Scale, Mini-Mental State Examination, and changes in cerebrospinal fluid, imaging, and plasma biomarkers.
After the successful acceptance of the POLARIS-AD Investigational New Drug (IND) and clinical trial applications in 11 countries, such as the United States, Korea, United Kingdom, Germany, France, Spain, Italy, Denmark, Netherlands, the Czech Republic, and now China, AriBio demonstrates its global dedication to developing innovative therapies for Alzheimer's disease.
Including China, a key player in the international pharmaceutical market, in the Phase 3 development of AR1001 allows AriBio to further enhance its influence in combating Alzheimer's disease. This trial aims to enroll approximately 1,150 participants across over 200 clinical centers in 11 countries.
AriBio’s CEO, Dr. Jai Jun Choung, expressed his enthusiasm for this milestone, stating, “We are elated to receive the IND approval for AR1001 in China. This major milestone underscores AriBio’s relentless effort to advance pioneering treatments for Alzheimer’s disease on a global stage. With IND approval now secured in all our targeted countries, including China, we are closer to offering hope to millions of patients globally.”
Looking ahead, AriBio plans to commence the Phase 3 clinical trial in China in the third quarter of 2024. Dr. Choung emphasized AriBio’s commitment to success, noting, “As the first Korean biopharmaceutical company to directly manage and conduct a global Phase 3 clinical trial of this magnitude, we approach our mission with pride and responsibility. We appreciate the support and attention from all participating countries and continue to pursue groundbreaking treatments for Alzheimer’s disease with unwavering determination.”
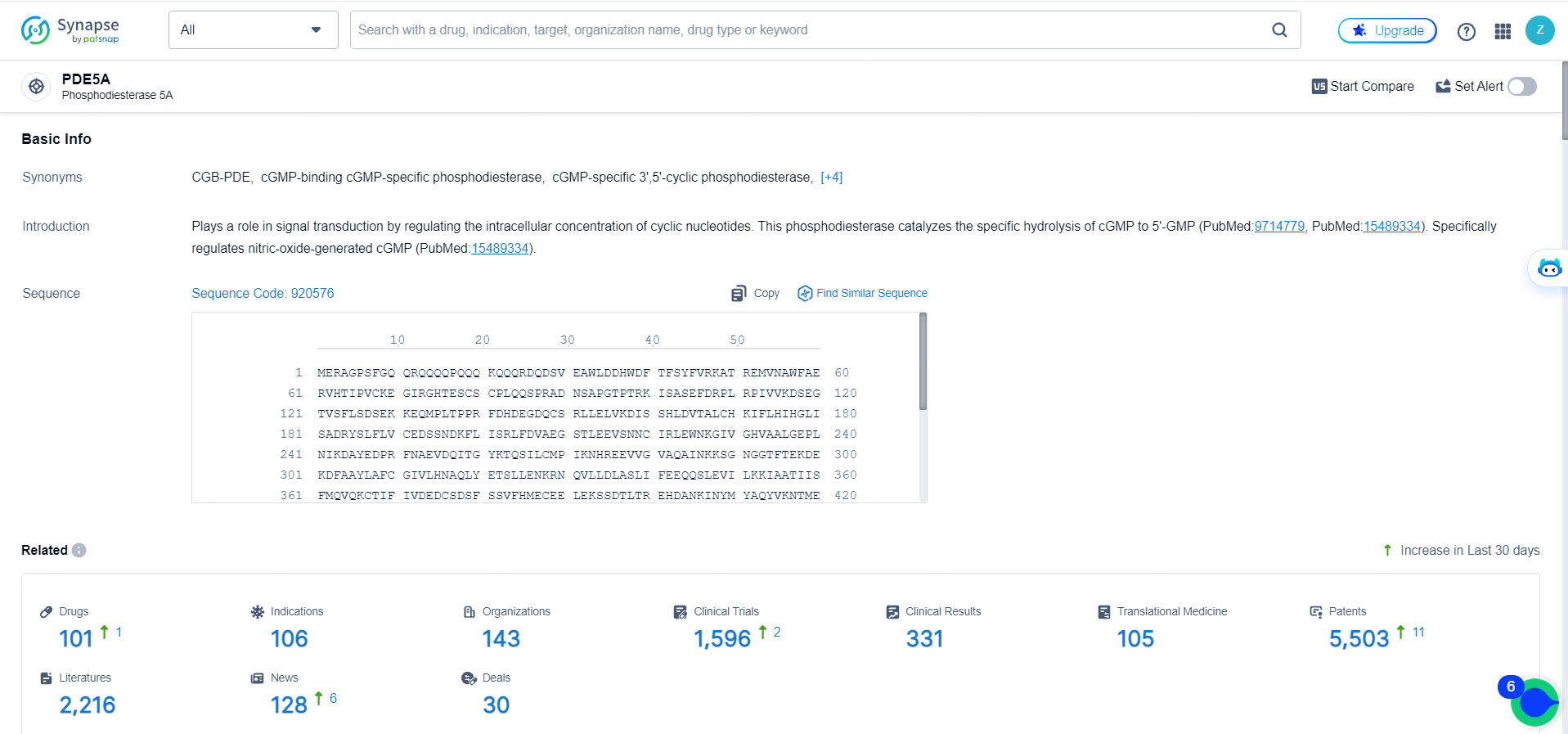
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 16, 2024, there are 101 investigational drugs for the PDE5 target, including 106 indications, 143 R&D institutions involved, with related clinical trials reaching 1596, and as many as 5503 patents.
AR1001 is a phosphodiesterase-5 (PDE5) inhibitor being developed as an investigational oral agent for the treatment of Alzheimer’s disease. Pre-clinical studies have confirmed neuroprotective effects of AR1001 via inhibiting neuron apoptosis and restoring synaptic plasticity, demonstrating multiple mechanisms of action for disease modifying effects that may ameliorate the course of AD.
Mirodenafil dihydrochloride represents a significant advancement in the field of biomedicine, particularly in the treatment of Alzheimer's Disease. Its progression to Phase 3 globally and IND approval in China demonstrates the potential for this small molecule drug to address unmet medical needs in the field of nervous system diseases.