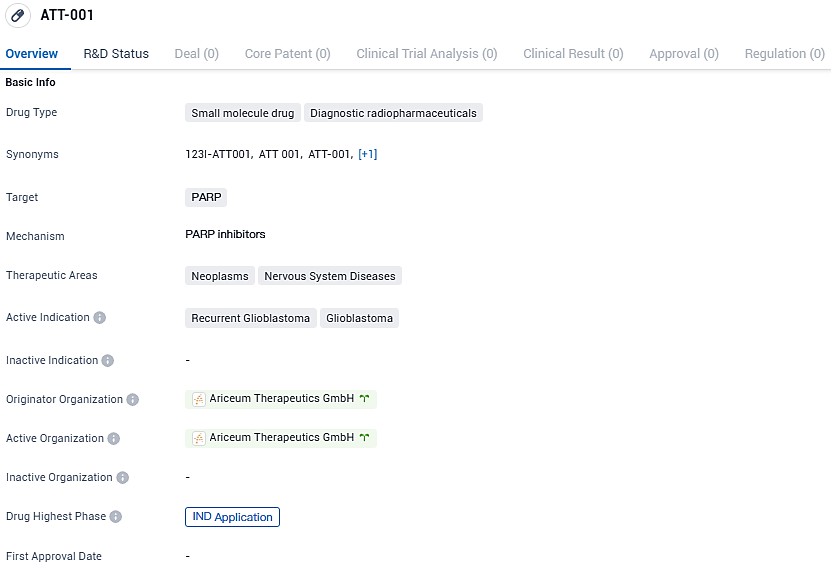
Ariceum Therapeutics has applied for a UK Clinical Trial Authorization to start testing its new Iodine-123 labeled PARP inhibitor in recurrent glioblastoma patients
Ariceum Therapeutics, an innovative life sciences company dedicated to creating new solutions in the radiopharmaceutical niche, has recently made public the submission of a formal request to the Medicines and Healthcare products Regulatory Agency in the United Kingdom. This request seeks authorization to initiate a preliminary clinical study, known as Phase 1, for its novel therapeutic agent, 123I-ATT001. This agent, characterized by its Iodine-123 tagged PARP inhibitor profile, is being investigated for its potential use in the clinical management of patients who are experiencing a resurgence of glioblastoma, a notoriously challenging type of brain cancer to effectively address.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Shortly following the acquisition of Theragnostics Ltd in June 2023, a private biopharmaceutical entity in the UK that initiated preclinical research on 123I-ATT001, Ariceum recently submitted an application. This submission marks Ariceum as the pioneer in funding a clinical investigation for the use of Auger therapy in the treatment of recurrent glioblastoma, a notably malignant brain tumor.
Manfred Rüdiger, the Chief Executive Officer at Ariceum Therapeutics, expressed his thoughts: "The prospect of 123I-ATT001, which is a precision therapy involving Auger electrons, has shown considerable potential in early-stage studies. We're excited about the opportunity to request approval for a clinical trial within the UK at this early stage post the Theragnostics assimilation into our operations at Ariceum Therapeutics. This effort is a significant stride not just for the potential healing of those living with glioblastoma but also for the advance of Ariceum in pursuing this potent form of molecular radiotherapy to potentially address a wider array of solid cancer types."
The therapeutic candidate, ATT001, delivers its radioactive component, Iodine-123, directly to the cancerous cells that utilize PARP, a critical enzyme for their self-repair mechanisms. Upon delivery, Iodine-123 releases low-energy Auger electrons which impart their destructive energy within a limited scope, hence destructing tumor cells whilst minimizing harm to surrounding healthy tissue. The choice of Iodine-123 for this purpose is beneficial due to its greater accessibility since it is produced in standard cyclotron facilities.
At the forefront of Ariceum's radiopharmaceutical innovations is the 177Lu-satoreotide tetraxetan, a targeted therapeutic that binds to and blocks the somatostatin type 2 receptors, frequently found in excess in neuroendocrine tumors and various aggressive cancer forms. Ariceum's research and development portfolio includes a radiolabelled PARP-inhibitor (ATT-001), anticipated to commence clinical trials in 2024. This inhibitor's development originates from the Theragnostics acquisition completed in June 2023.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
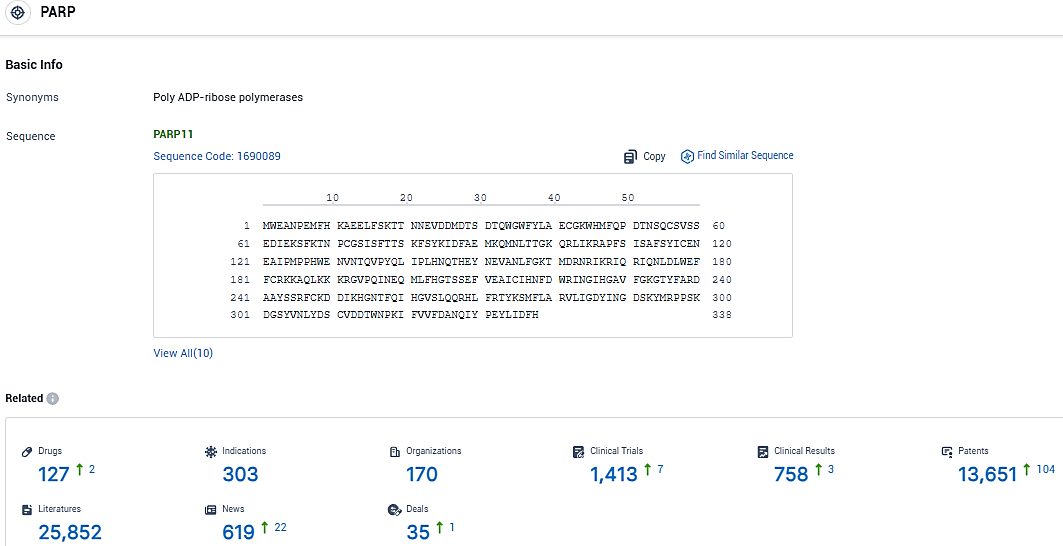
According to the data provided by the Synapse Database, As of January 16, 2024, there are 127 investigational drugs for the PARP target, including 303 indications, 170 R&D institutions involved, with related clinical trials reaching 1413, and as many as 13651 patents.
ATT-001 is a small molecule drug categorized as a diagnostic radiopharmaceutical that targets PARP. It is indicated for the treatment of recurrent glioblastoma and glioblastoma, both of which are neoplasms affecting the nervous system. The drug is currently in the IND application phase of development.