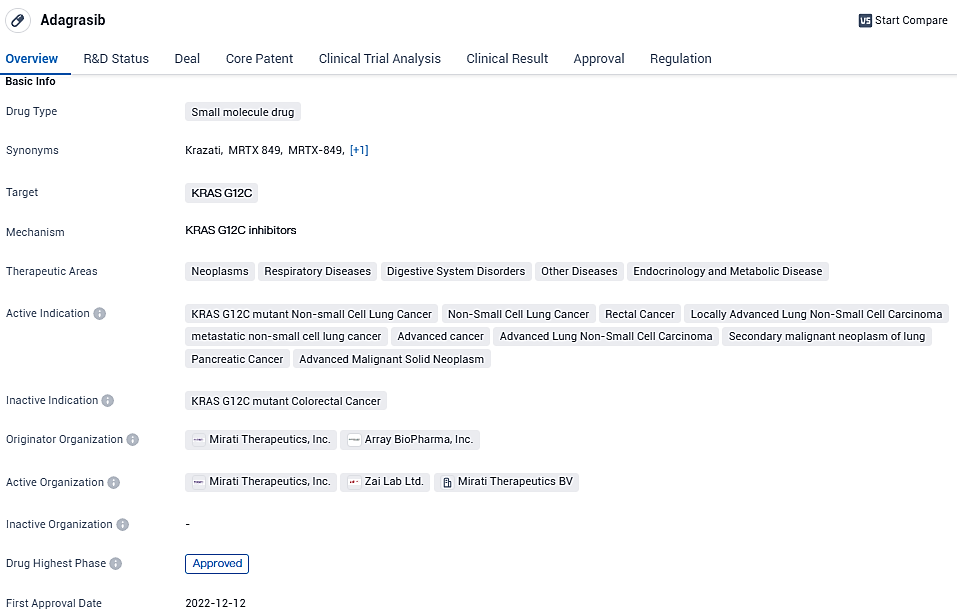
EU Sanctions KRAZATI (adagrasib) as a Selective Therapy for Advanced NSCLC Sufferers Exhibiting KRAS G12C Alteration
Mirati Therapeutics, Inc., an enterprise engaged in biotechnology at the commercialization phase, revealed today that the European Commission has approved a provisional marketing permission for their drug, KRAZATI® (adagrasib). This authorization paves the way for the use of adagrasib as a selective therapeutic intervention for adult individuals suffering from advanced non-small cell lung cancer characterized by the KRAS G12C mutation, particularly for those whose disease has advanced following a minimum of one prior systemic treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
KRAZATI has shown a promising balance of effectiveness and safety according to the data from the second phase's pivotal segment of the KRYSTAL-1 trial. During this study, 116 individuals exhibiting advanced NSCLC with the KRASG12C mutation, all of whom had been previously treated with a platinum-containing drug framework and immunotherapy involving an immune checkpoint inhibitor, were given KRAZATI at a dose of 600 mg through oral administration, twice every day. The main parameters for measuring success included the verified Overall Response Rate (ORR) and Duration of Response (DOR), and these were assessed with a blind independent central analysis using established criteria for evaluating solid tumor responses.
"KRAZATI has established its role as an effective and manageable option for individuals diagnosed with advanced KRASG12C-mutant NSCLC, and its endorsement paves the way for additional therapeutic interventions," stated Martin Reck, MD, PhD at the Lung Clinic Grosshansdorf in Germany. "KRAZATI, due to its unique characteristics, can exert a significant influence on the treatment of lung cancer. This authorization enables medical professionals to design more personalized treatment strategies for their patients."
KRAZATI's conditional marketing approval is now recognized in every one of the 27 European Union countries as well as in Iceland, Norway, and Liechtenstein. This certification is the result of the favorable viewpoint issued by the European Medicines Agency's Committee for Medicinal Products for Human Use in November 2023.
"This marks a significant moment for patients across the European Union who are up against this particularly challenging type of cancer, as they now have access to a distinct and potentially superior therapeutic alternative," said Charles Baum, M.D., PhD, the founding director, president, and CEO at Mirati Therapeutics, Inc. "Mirati is committed to continuing to work closely with European Union nations to ensure that all eligible patients can have extensive access to this treatment."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
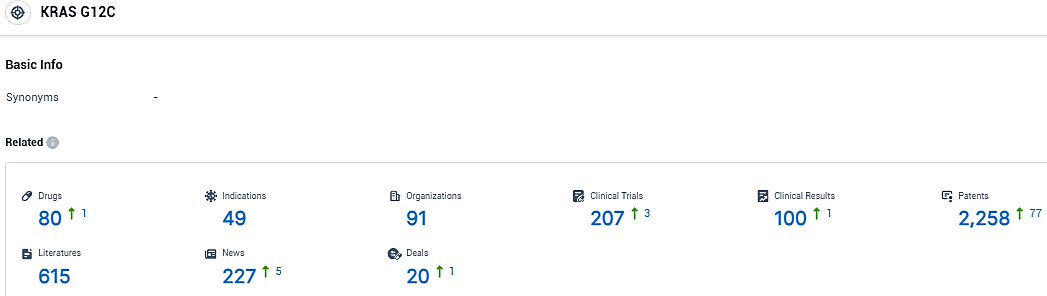
According to the data provided by the Synapse Database, As of January 15, 2024, there are 80 investigational drugs for the KRAS G12C target, including 49 indications, 91 R&D institutions involved, with related clinical trials reaching 207, and as many as 2258 patents.
Adagrasib continues to be evaluated as monotherapy and in combination with other anti-cancer therapies in patients with advanced KRAS G12C-mutated solid tumors, including NSCLC, colorectal cancer, and pancreatic cancer. The FDA provided KRAZATI Accelerated Approval, allowing for the approval of drugs that treat serious conditions, and that fill an unmet medical need based on surrogate endpoints.