Aruna Bio's AB126 exosome therapy has gained FDA approval for its IND application
Aruna Bio, Inc., a leading innovator in creating neural exosome-derived treatments targeting neurodegenerative diseases, has declared that the U.S. Food and Drug Administration approved the initiation of clinical trials for their primary therapeutic candidate, AB126.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
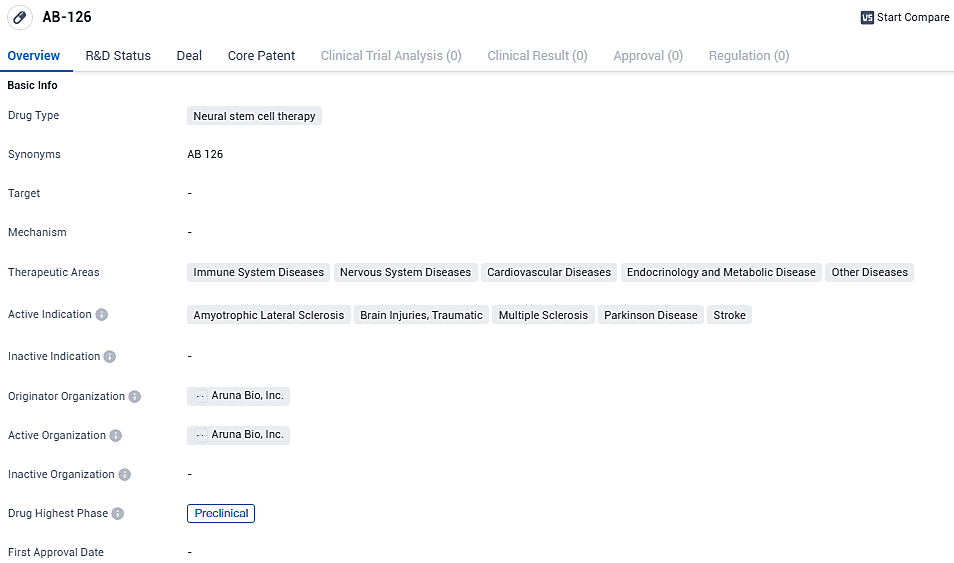
The go-ahead has been granted for the commencement of the phase 1b/2a research study on acute ischemic stroke, anticipated to kick-off during the initial six months of 2024. AB126, a neural-derived exosome that remains unaltered, possesses the innate capability of penetrating the blood-brain barrier and demonstrates potential anti-inflammatory and neuroprotective effects.
"The endorsement from the FDA is tremendously encouraging," proclaimed Steven Stice, Ph.D., the Co-Founder and Chief Scientific Officer at Aruna. "It marks AB126 as the inaugural exosome therapy trialed in humans for a neurological condition and highlights our platform's therapeutic promise."
"Moreover, exosome production hinges on the preservation of cellular conditions, which is a critical element. We are eager to apply our GMP manufacturing skills in-house as we progress in clinical research," Stice noted, "while also broadening AB126's scope to further medical conditions, like amyotrophic lateral sclerosis, and advancing the vast potential of our neural exosome technology for solving prevalent obstacles in central nervous system therapies."
Aruna's specialized GMP facility is designed for the crafting of clinical-standard batches, ensuring supreme scalability and batch-to-batch reliability. The advanced purification methods applied result in meticulous oversight over essential qualities. The development of methodical analytical procedures alongside thoroughly defined assays is crucial for guaranteeing therapeutic uniformity.
Dr. Sean Savitz, the Primary Investigator and a Professor of Neurology, also serving as Director of the Institute for Stroke and Cerebrovascular Disease at UTHealth Houston, remarked, "We are eager to advance from preclinical insights which suggest that AB126 could lessen neuro-inflammation, potentially aiding in neuroprotection, as well as advancing neuro-regeneration."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
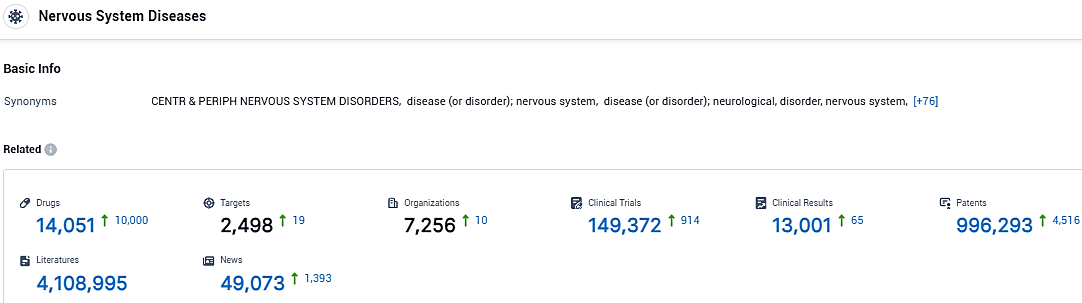
According to the data provided by the Synapse Database, As of January 23, 2024, there are 14051 investigational drugs for the Nervous System Diseases, including 2498 targets, 7256 R&D institutions involved, with related clinical trials reaching 149372, and as many as 996293 patents.
AB-126 is a neural stem cell therapy drug being developed by Aruna Bio, Inc. It targets a range of therapeutic areas, including immune system diseases, nervous system diseases, cardiovascular diseases, endocrinology and metabolic diseases, and other diseases. The active indications for AB-126 include ALS, brain injuries, multiple sclerosis, Parkinson's disease, and stroke. While still in the preclinical phase, AB-126 shows promise in potentially providing regenerative and therapeutic benefits for patients.