Exploring the Latest ETA Antagonists Deal by SanReno Therapeutics: A Guide to Rapidly Accessing Transaction Insights
On January 5, 2024, Novartis Pharma announced an agreement for the acquisition of SanReno Therapeutics, marking a significant step in strengthening its kidney disease portfolio. According to the agreement, Novartis will acquire the remaining shares of Sanreno. Following the completion of the transaction, Sanreno will be fully integrated into Novartis China. The core assets of Sanreno include two drugs in the clinical development phase, namely atrasentan and zigakibart (BION-1301), both aimed at treating IgA nephropathy. The following text will focus on introducing atrasentan.
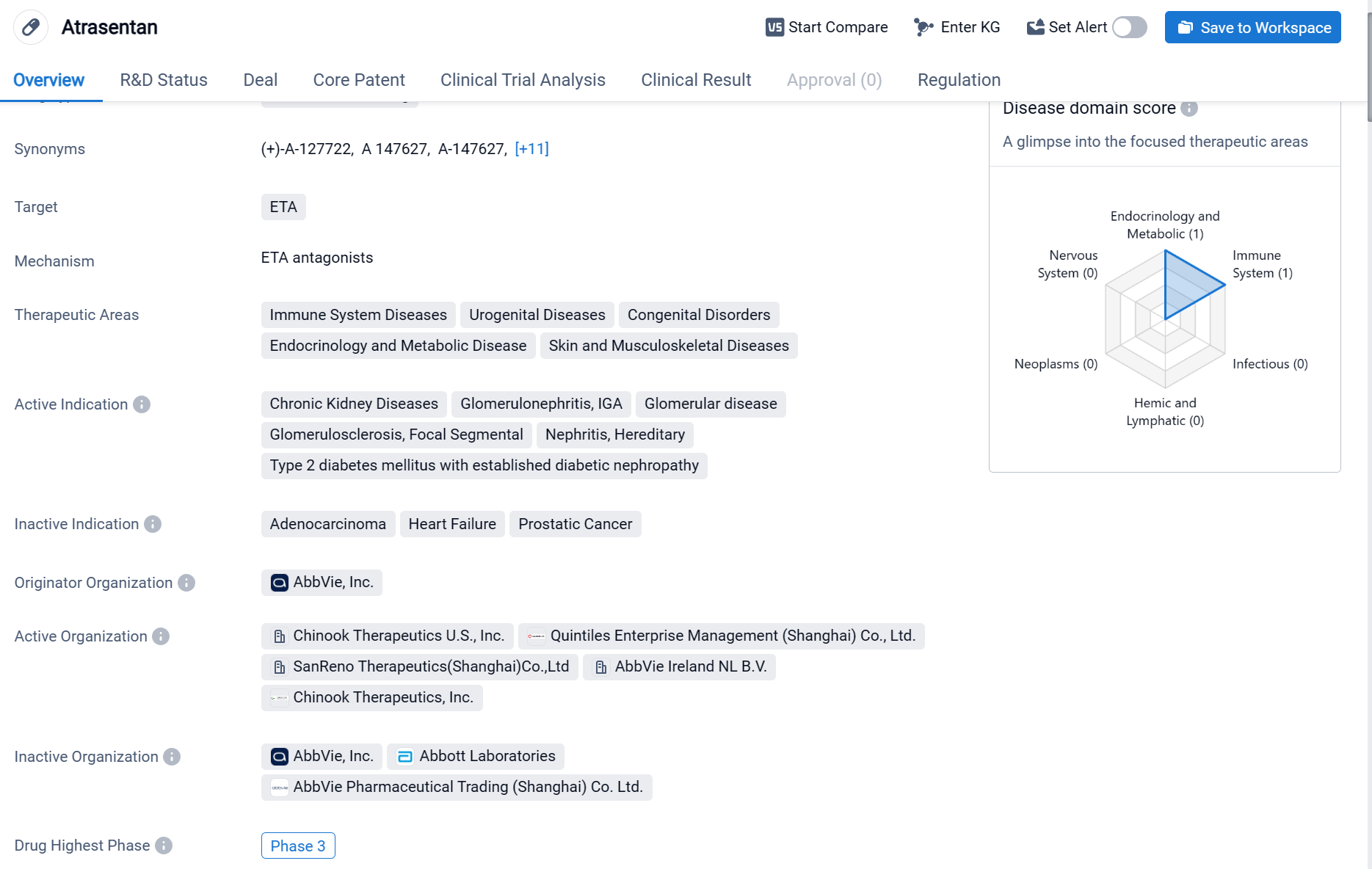
About Atrasentan
Atrasentan is a small molecule drug that targets the ETA receptor. It is being developed for the treatment of various therapeutic areas including immune system diseases, urogenital diseases, congenital disorders, endocrinology and metabolic disease, as well as skin and musculoskeletal diseases. The drug has shown potential in treating chronic kidney diseases, glomerulonephritis (specifically IGA glomerular disease), glomerulosclerosis (focal segmental), nephritis (hereditary), and type 2 diabetes mellitus with established diabetic nephropathy. Click the image below to directly embark on the exploration journey with the Atrasentan!
On October 30, 2023, Novartis announced the positive top-line results from the interim analysis of the ongoing Phase III ALIGN study (NCT04573478) for atrasentan, which achieved its primary endpoint 1 during the 36-week interim analysis. Novartis plans to seek opportunities for accelerated approval in the United States in 2024 based on the results of the interim endpoint analysis on proteinuria.
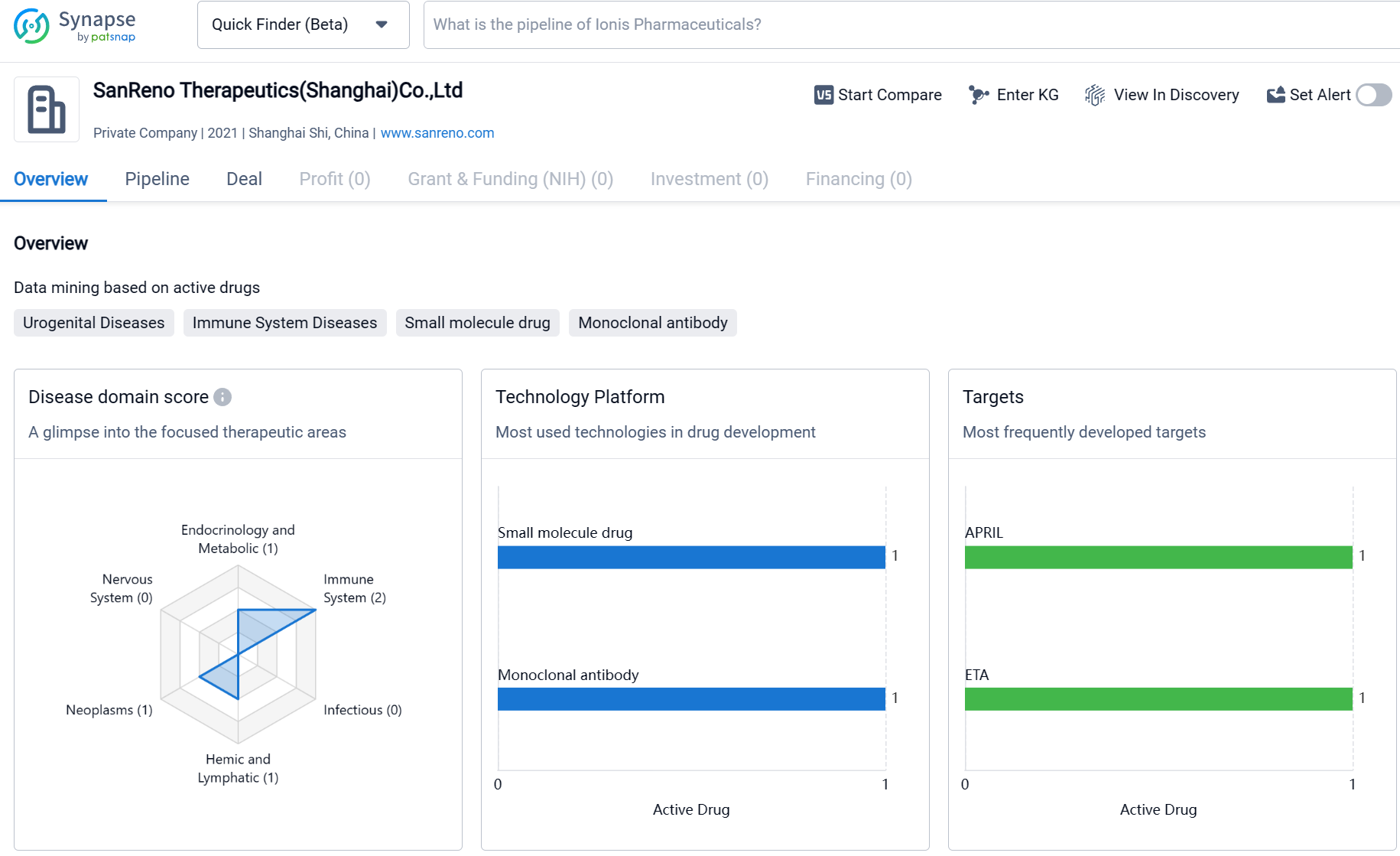
About SanReno Therapeutics (Shanghai)
SanReno Therapeutics (Shanghai) Co., Ltd is a biomedicine organization founded in 2021, with a focus on developing therapeutics for various therapeutic areas. The company has a diverse portfolio of drugs targeting immune system diseases, urogenital diseases, cardiovascular diseases, and other areas. It has made progress in advancing its drug candidates through clinical development phases, with drugs in Phase 2 and Phase 3. However, further information is needed to fully assess the company's business development prospects and potential for success in the pharmaceutical industry.
How to get the latest progress on drug deals?
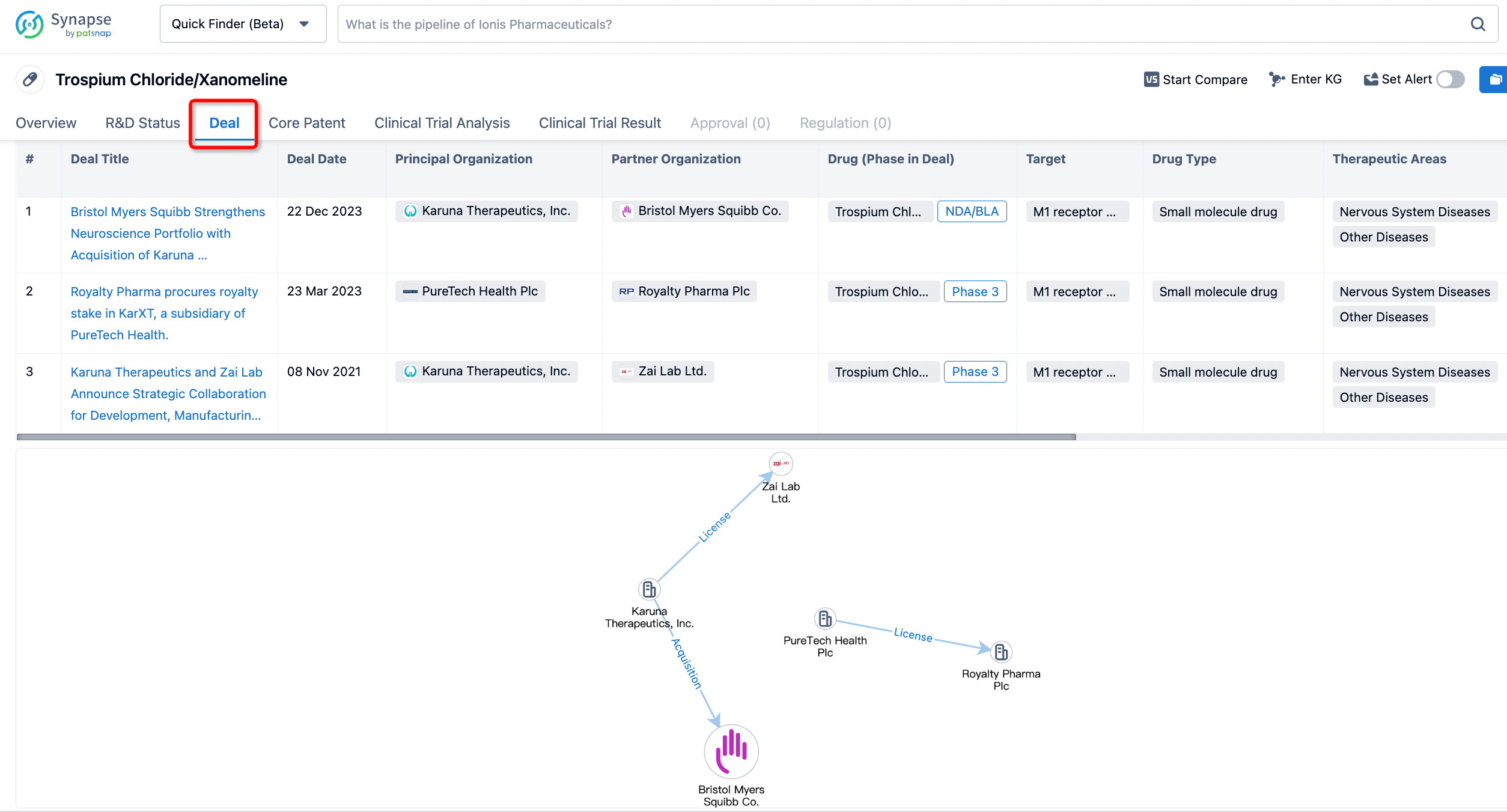
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
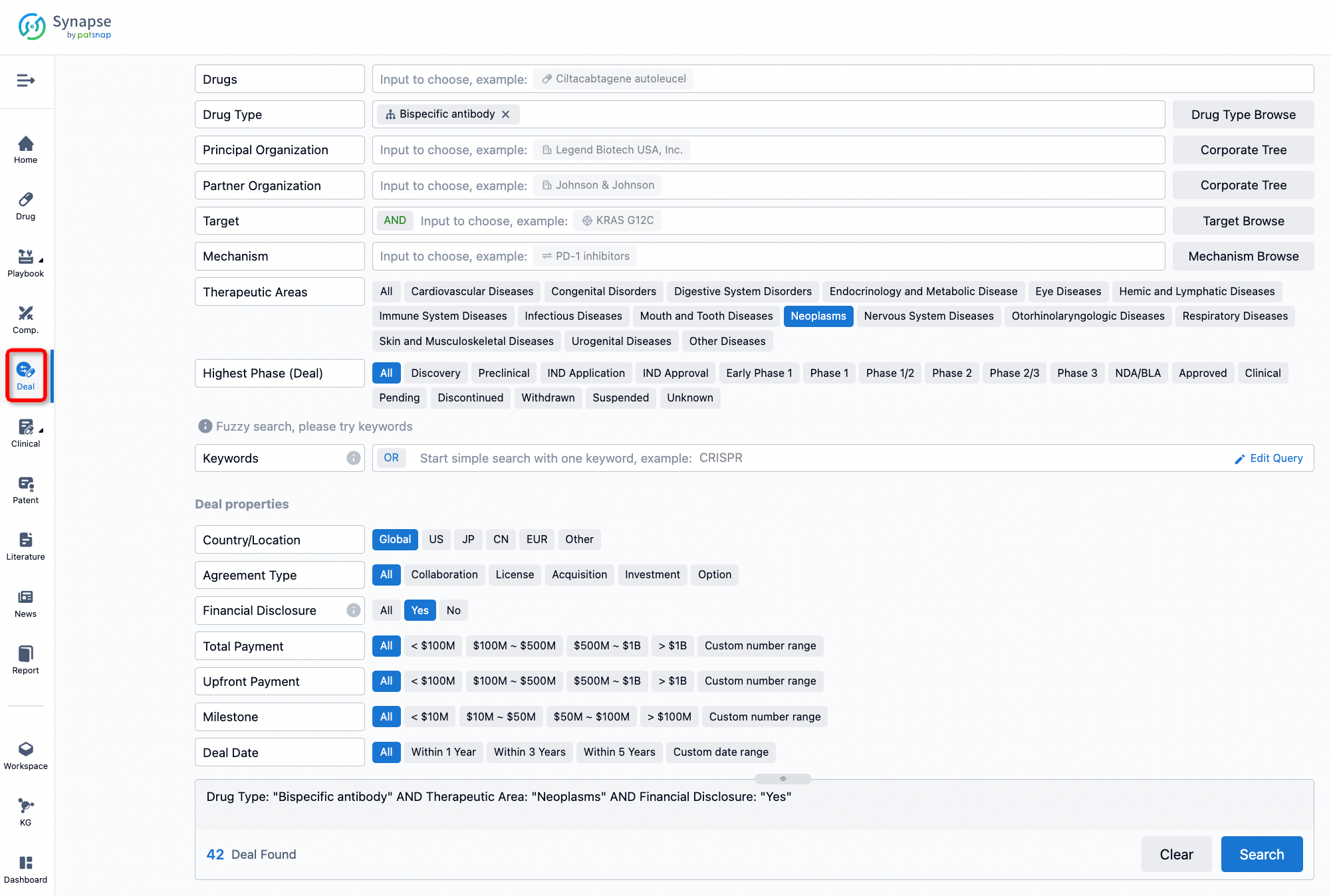
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
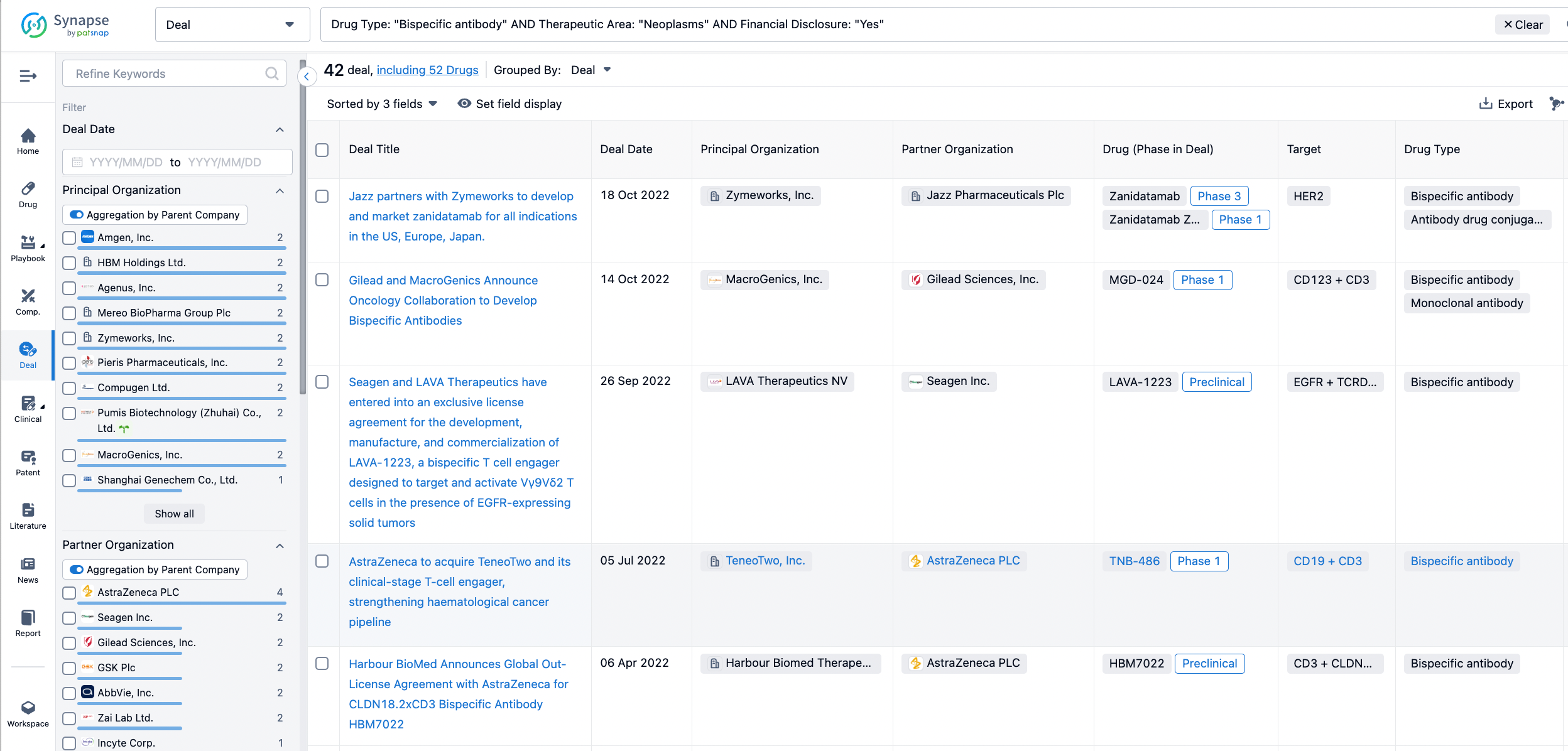
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
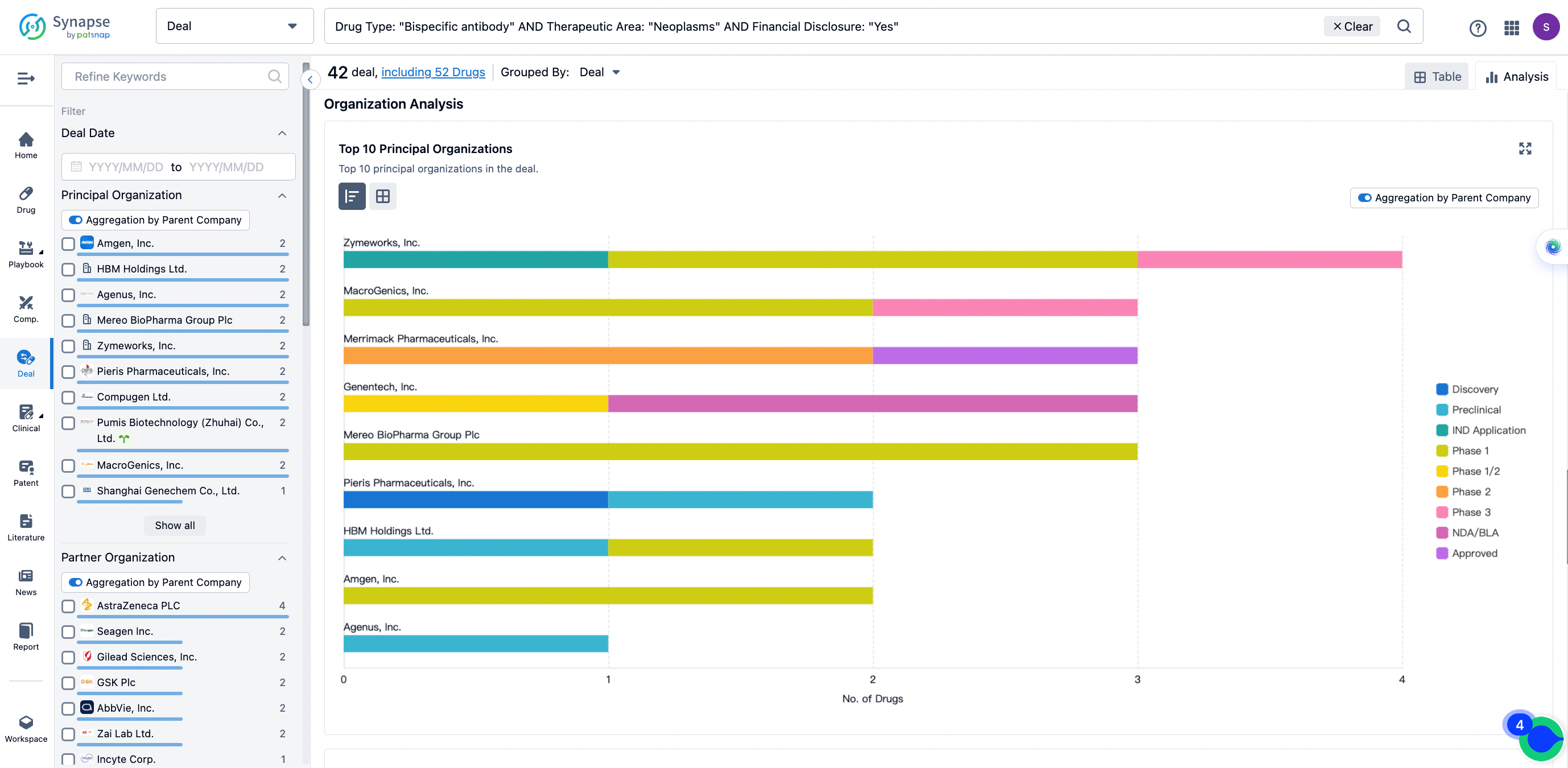
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
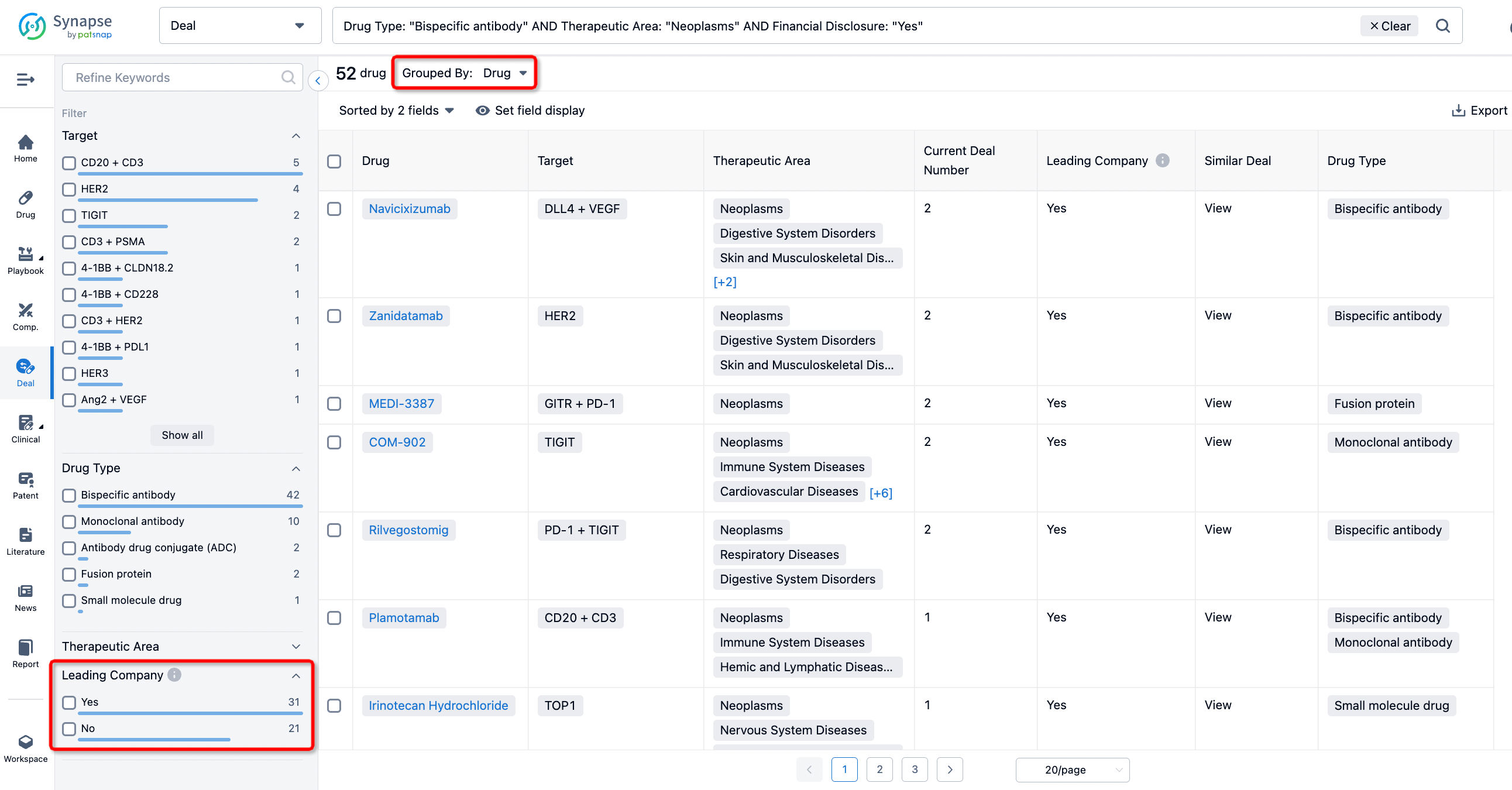
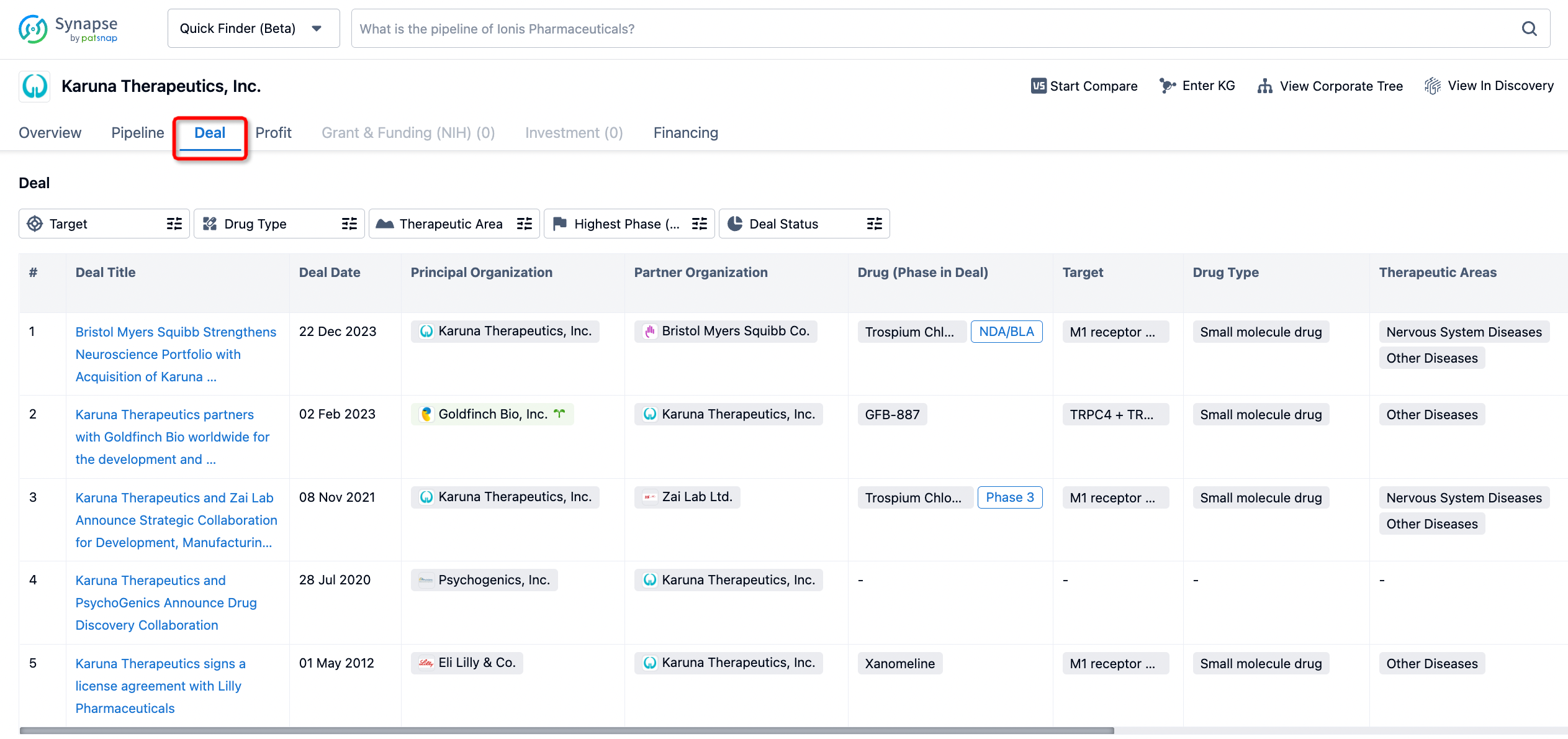
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!