Sarepta's EMERGENE Phase 3 Trial on SRP-9003 for LGMD 2E/R4 Begins Participant Enrollment
Sarepta Therapeutics, Inc., a pioneering company in the field of targeted genetic treatments for uncommon illnesses, has declared that participant selection has commenced for the clinical trial titled SRP-9003-301. Referred to as EMERGENE, the 9003-301 trial is a Stage 3 global clinical trial with an open-label design that is evaluating SRP-9003 (bidridistrogene xeboparvovec) in patients suffering from limb-girdle muscular dystrophy Type 2E, also known as beta sarcoglycanopathy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
We are delighted to convey the ongoing advancements we've made with SRP-9003, an uncommon LGMD that currently lacks comprehensive treatment options beyond simply addressing symptoms. Preliminary outcomes from the trials of SRP-9003 have shown substantial levels of protein synthesis occurring at the 12-week mark and persisting for up to two years post-administration, alongside observable improvements in patient function that help decelerate the disease's advancement,” explained Louise Rodino-Klapac, Ph.D., the senior vice president and principal scientific leader with the added responsibility of overseeing research and development at Sarepta Therapeutics.
Dr. Rodino-Klapac added, “Beyond the direct impact for patients with LGMD2E, the EMERGENE initiative is set to provide valuable insights for the clinical strategy of additional LGMD projects within the portfolio of Sarepta Therapeutics. It also paves the way for establishing effective regulatory strategies that could accelerate the approval processes for gene therapies aiming to address extremely rare diseases.”
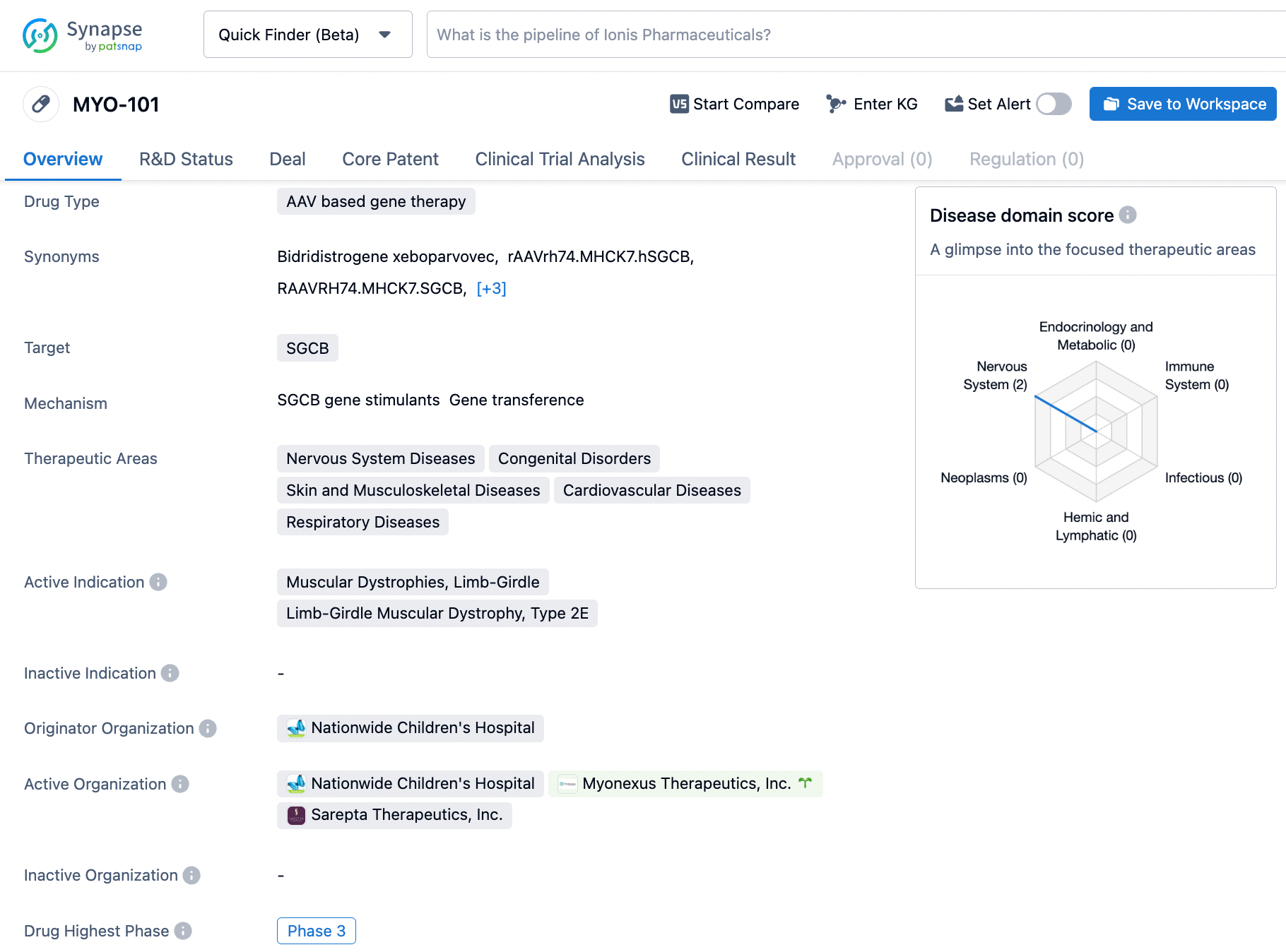
The investigational gene therapy SRP-9003, also known as bidridistrogene xeboparvovec, harnesses the AAVrh74 vector for its administration, which has been tailored for systemic and effective delivery to the skeletal, diaphragmatic, and heart muscles, rendering it an optimal therapeutic agent for various muscular conditions. SRP-9003's design includes the transfer of a complete beta-sarcoglycan gene and is driven by the MHCK7 promoter, a promoter selected for its demonstrated strength in cardiac expression.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
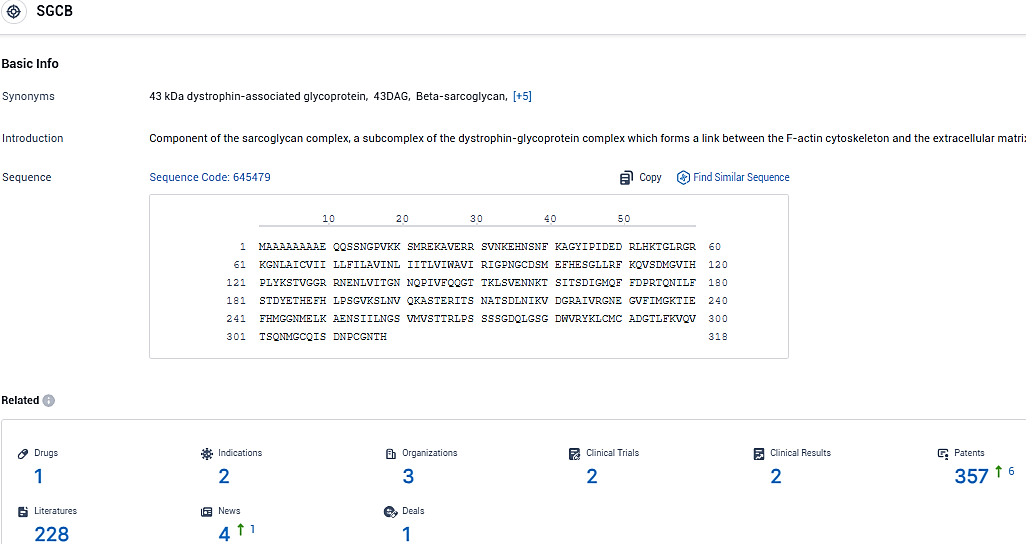
According to the data provided by the Synapse Database, As of January 19, 2024, there are 1 investigational drugs for the SGCB target, including 2 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 357 patents.
MYO-101 targets the SGCB gene and is primarily indicated for Limb-Girdle Muscular Dystrophy, Type 2E. However, its therapeutic areas suggest potential applications in various diseases affecting the nervous system, musculoskeletal system, skin, cardiovascular system, and respiratory system. With its current status in Phase 2, MYO-101 shows promise in the field of biomedicine for the treatment of these conditions.