CARsgen's CT011 Granted NMPA Clearance for Phase IIIa Liver Cancer Trial
CARsgen Therapeutics Holdings Limited, a firm dedicated to pioneering chimeric antigen receptor (CAR) T-cell treatments targeting both blood cancers and solid neoplasms, revealed that their autologous CAR T-cell therapy, CT011, designed to target Glypican-3 (GPC3), has successfully secured Investigational New Drug authorization from the National Medical Products Administration. This approval enables CT011 to be tested in clinical trials for individuals with GPC3-positive hepatocellular carcinoma at stage IIIa, particularly those facing a significant risk of the disease returning following surgery.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Raffaele Baffa, holding the position of Chief Medical Officer at CARsgen Therapeutics, has stated, "GPC3 was initially chosen as a promising marker for the deployment of CAR T-cell treatment, which we then advanced into clinical testing phases for combating HCC. Clinical anecdotes reveal that individuals suffering from severe hepatocellular carcinoma have attained over seven years of survival without disease recurrence. Our efforts will persist in investigating CAR-T's capabilities against solid malignancies while aiming to introduce novel therapeutic avenues for those in need."
The experimental therapy known as CT011, a GPC3-specific CAR T-cell treatment designed for hepatocellular carcinoma, is an investigational product engineered from a patient's own T cells. It achieved a groundbreaking milestone by acquiring IND approval from the NMPA in 2019 as China's inaugural CAR T-cell therapy for solid tumors with the GPC3 marker. CARsgen has successfully reached full participation in a Phase I study in the Chinese medical landscape.
The investigational agent CT-011 demonstrates significant potential as an innovative approach to treat not only advanced hepatocellular carcinoma but also those cases expressing GPC3. Nevertheless, as it remains in the initial phase of clinical research, a comprehensive evaluation is necessary to authentically determine its therapeutic effectiveness and safety for patients.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
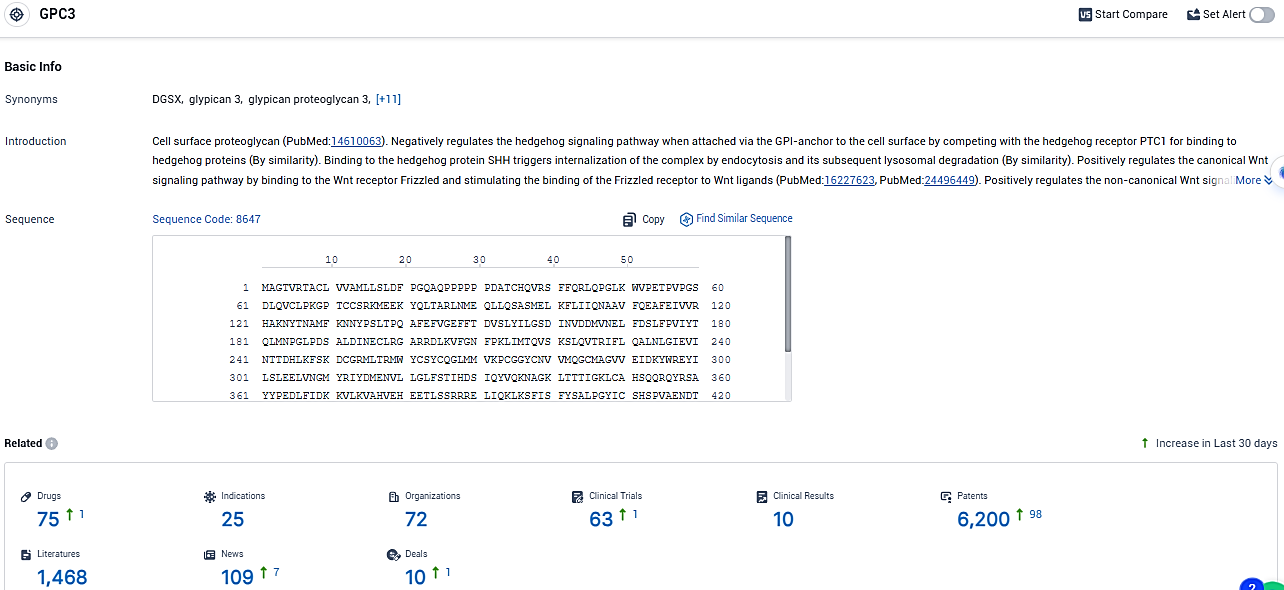
According to the data provided by the Synapse Database, As of January 19, 2024, there are 75 investigational drugs for the GPC3 target, including 25 indications, 72 R&D institutions involved, with related clinical trials reaching 63, and as many as 6200 patents.
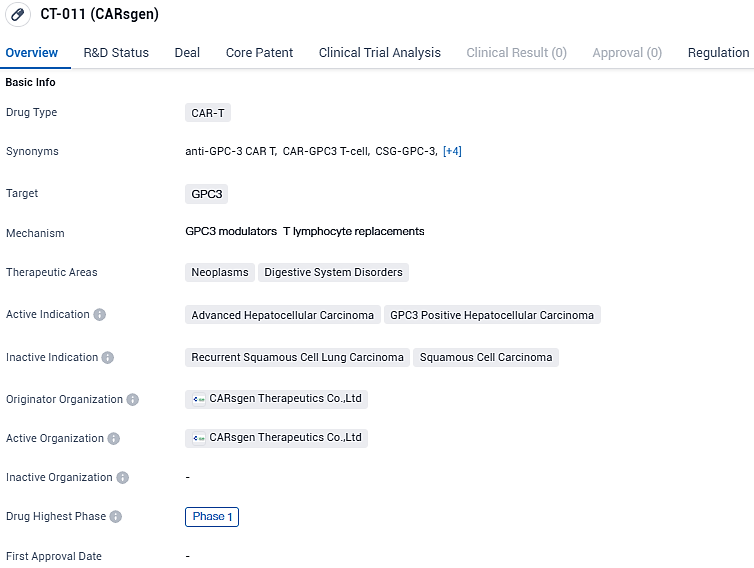
CT-011 is a CAR-T drug that targets GPC3 and is primarily focused on treating neoplasms and digestive system disorders. Its active indication is advanced hepatocellular carcinoma and GPC3 positive hepatocellular carcinoma. Currently, CT-011 is in Phase 1 of clinical trials, both globally and in China. This means that the drug is still in the early stages of development and is being tested for its safety, dosage, and potential side effects. Phase 1 trials typically involve a small number of participants and aim to determine the appropriate dosage and evaluate the drug's safety profile.